BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://cjns.gums.ac.ir/article-1-80-en.html
2- Resident of Emergency Medicine, Tabriz University of Medical Sciences, Tabriz, Iran
3- Graduated from School of Medicine, Faculty of Medicine, Urmia University of Medical Sciences, Urmia, Iran
ABSTRACT
Background: Peripheral nerves may be damaged during an injury and its current standard treatment is using an autologous nerve.
Objectives: The purpose of this experimental study is to evaluate and compare the histological results of nerve regeneration after using the eggshell membrane (ESM) guidance channel with autograft.
Materials and Methods: Thirty adult male rats were divided into three experimental groups: ESM guidance channel, autograft, and sham surgery. The decalcifying membrane of egg rotated over the Teflon mandrel and dried at 37°C. A 10 mm nerve segment of left sciatic nerve was cut and removed. In ESM group, the ends of the sciatic nerve were telescoped into the nerve guides. In autograft group, the nerve segment was reversed and used as an autologous nerve graft. At 90 days after surgery, all animals were evaluated by histological and immunohistochemical assessment.
Results: The diameters of regenerated myelinated fibers were 5.24±2.14 µm for the ESM group, and 5.89±2.99 µm for the autograft group. The number of myelinated axons regenerated in the ESM group (9824±218 nerve fibers) was significantly greater than autograft group (7865±314 nerve fibers) (p<0.05).
Conclusion: These findings demonstrate that ESM effectively enhances nerve regeneration in injured rat sciatic nerve.
Keywords: Nerve Regeneration; Nerve Fibers, Myelinated; Rats
Introduction
Neural tissue repair and regeneration strategies have received a great deal of attention it directly affects the quality of the patient's life (1). A large gap in the peripheral nerve will not permit effective regeneration unless a grafting conduit is used to bridge the defect (2). The current gold standard for the clinical treatment of severe peripheral nerve damage involves using an autologous nerve to bridge the defect in injured nerve (3). This method has been shown to be effective, but has the several disadvantages, including an extra incision for removal of a healthy sensory nerve ultimately resulting in a sensory deficit at the donor site (4-5), also neuroma and scar tissue are common (6). In an effort to overcome these limitations (7), the use of nerve guidance channels (NGCs) to bridge the gap between severed nerve ends is being extensively explored (3). Currently a vast amount of research was being pursued to engineer the ideal NGCs that can promote both sensory and motor functions (8).
The eggshell membrane (ESM) is a biopolymer network that may have potential applications in biomedicine (9). The chicken eggshell and its membranes are an inexpensive and abundant waste material that exhibit interesting characteristics for many potential applications (10). The ESM retains albumen and prevent penetration of bacteria (11). The discovery of ESM as a natural source of combined glucosamine, chondroitin, and hyaluronic acid has prompted treatment for osteoarthritis (12). The ESM has a high content of bioactive components, as well as properties of moisture retention and biodegradability, which have potential use for clinical, cosmetic, nutraceutical and nanotechnology applications (10). The soluble ESM protein has applications in tissue engineering (13). ESM is suitable for the adherence of stromal cells and a biological dressing for burn (14,15). Based on the composition and various applications with ease of processing may have a great potential in clinical practice, for example, as wound dressing and tissue engineering scaffold (16). ESM is non-toxic and biodegradable (17,18). The hen eggshell membrane protects the fetus just as the human amniotic membrane does (14). It may have a great potential nerve guide for studies of axonal regeneration in the peripheral nervous system (10). Our previous study showed that ESM promotes functional recovery in injured sciatic nerve of rat (19).
The purpose of this experiment was to evaluate the outcome of nerve regeneration by histology and immunohisto-chemistry testing across the ESM guidance channels in comparison with autograft.
Materials and Methods
Animals
Thirty adult male Sprague-Dawley rats weighing 275 to 300 g, were randomized into three groups, including (i) ESM guidance channels (n=10), (ii) nerve autograft (n=10), (iii) sham surgery (n=10). The experimental procedures were approved by the ethical committee of Urmia University of Medical Sciences. The left sciatic nerve was used as experimental side and the other side to serve as the control.
Preparation of ESM Guidance Channel
On previous paper we described the preparation of the ESM guidance channel. Briefly, four fresh hen eggs were opened at their blunt ends (19). The fluid contents were poured out. The remaining calcareous cups were submerged completely in 5% acetic acid for about 8 days until the membranes were soft and were completely free of brittle eggshell remnants. The sacs were taken and cut into four pieces (20). These films were immersed in phosphate buffered saline, pH 7.4 for a period of 30 minutes and rotated over the Teflon mandrel manually under sterile conditions to have a longitudinal orientation. The Teflon mandrel along with the so formed ESM conduit was removed. The ESM conduits were then individually packed and sterilized with ethylene oxide for 24 hours at room temperature (18). The conduits were stored indefinitely in the refrigerator after sterilization, and remove a few as needed for each experiment. The ESM nerve conduit measured 2 mm in inner diameter, 12 mm in length, and wall thickness of 0.6 mm.
Surgical Procedure
The animals were anesthetized using ketamine (90 mg/kg) and xylazine (10 mg/kg) intraperitoneally. The left sciatic nerve of the rat was exposed through a 4 cm long skin incision on the posterolateral of thigh and by a gluteal muscle splitting incision. The operation was terminated at this point in sham-operated group. A 10 mm nerve segment was removed proximal to the tibial and the peroneal nerve bifurcation and leaving a gap of approximately 10 mm due to retraction of the nerve stumps. In ESM group, both the proximal and distal cut ends of the sciatic nerve were telescoped into the ends of the nerve guides and fixed with a single 10-0 nylon epineurial suture. Before inserting the distal stump, the lumen of the guide was filled with sterile, physiologic normal saline, in order to prevent trapping of air bubbles within their lumens and the end of the guide was sealed with petrolatum jelly.
In autograft group, the nerve segment was reversed and used as an autologous nerve graft. The muscle was closed with 4-0 Dexon sutures, and the skin was closed with 3-0 nylon sutures.
All were given a single weight related subcutaneous dose of an antibiotic (penicillin-procaine, 400000 IU) the day before the operation. The animal allowed to recover with free access to food and to water containing 0.64 mg/ml acetaminophen for the first 48 hours postoperative.
Histological Examination
In all groups 90 days after surgery, middle cable in ESM group, the midpoint of autograft nerve, and sham surgery (left side) and intact nerve (right side) were removed. The samples were fixed at 10% buffered formalin, dehydrated, and embedded in
paraffin blocks. In each animal, the nerve segment was entirely sliced as serial 5-µm across the transverse axis, but only 10 sections and five microscopic fields in each section were randomly selected for analysis. The sections were mounted and stained with toluidine blue and examined via light microscopy at ×40 magnification. The first step of the morphometric analysis consisted of capturing the image of each individual fascicle. The next step, the mylinated fibers were counted, and the myelinated fiber diameter and myelin sheath thickness measurements were performed in each nerve cross-section with the aid of (OLYSIA Biorefort, Olympus, Japan) morphometric analysis system. The total mylinated fibers and fiber diameter or myelin sheath thickness corresponded to the sum of them in each fascicle. The mean value was calculated on the 10 slices of each nerve segment. All measurements were made by a single, skilled observer.
Immunohistochemistry
In this study, anti S-100 (Dako, 1:200 dilution) was used as a marker for myelin sheath. Briefly, specimens prior to immunohistochemistry were post-fixed in a solution containing 4% Paraformaldehyde for 2 hours. The tissue samples were embedded in paraffin and cut into 5 µm thick sections. According to the instructions of immunohistochemical staining kits, after that the sections were incubated with normal swine serum (Dako, 1:50) for blocking of non-specific immunoreactions; sections were incubated in S-100 protein antibody solutions for 1 hour at room temperature and then washed three times with PBS. They were incubated in biotinylated anti-mouse rabbit IgG solution for 1 hour at room temperature
and washed with PBS for 5 min, 3 times in all. Horseradish peroxidase-labelled secondary antibody solution was added to the sections. The sections were incubated for 10-20 minutes at room temperature and washed with PBS for 5 minutes, 3 times in all.
Finally, the sections were fully washed under running tap water and counter-stained with hematoxylin and mounted. The results of immunohistochemistry were examined under a light microscope.
All experiments were conducted in accordance with the Guide for Care and Use of Laboratory Animals (National Institute of Health Publication No.80-23, revised 1996).
Statistical analysis
Statistical analysis was done with 95% confidence level using the SPSS (version 16.0 for Windows) software. All data were analyzed by one way ANOVA and followed by Tukey post test comparison. All data are presented as mean±SD (p<0.05) was taken as being statistically significant.
Results
The microscopic examination on the cross-section of the nerve guide showed that the conduit wall was solid and impact, although in some areas of the conduit slight micro air bubbles were seen.
In ESM group, the nerve cables contained fascicles of axons. Blood vessels were observed throughout the regenerated tissue. The neural tissue was surrounded by an epineurium composed of several layers of circumferentially arranged fibroblast and collagen fiber layers. A layer of macrophages could be seen on the outer surface of the ESM conduits. A fibrous layer up to 20 µm thick was adjacent to the ESM conduit to the outer surface. The regenerated cables were centrally located in the conduit. All cables were circular in shape and surrounded by a thin neoepineurium and contained numerous Schwann cells, blood vessels, and myelinated axons within microfascicles. Mast cells were occasionally seen within the regenerated cable. The autograft group showed larger areas of connective tissue between the axons in a poorly organized architecture, not resembling the normal endoneurial layers. The myelinated axons were easily identified by their myelin sheaths. All myelinated axons present in each nerve cross-section were counted.
Myelinated axon numbers were significantly greater for the ESM group (9824±218 nerve fibers) vs. the autograft group (7865±314 nerve fibers) (p<0.05). The average myelin sheath thickness for autograft group (0.54±0.21 µm) was greater compared to the ESM group (0.46±0.32 µm), but the difference was not significant (p>0.05).
The regenerated nerves in all groups had smaller diameter axons and thinner myelin sheath than the normal nerve. Histological examination of the ESM group although showed a number of axons similar to normal controls, the diameters of the axons were smaller than normal (Table 1).
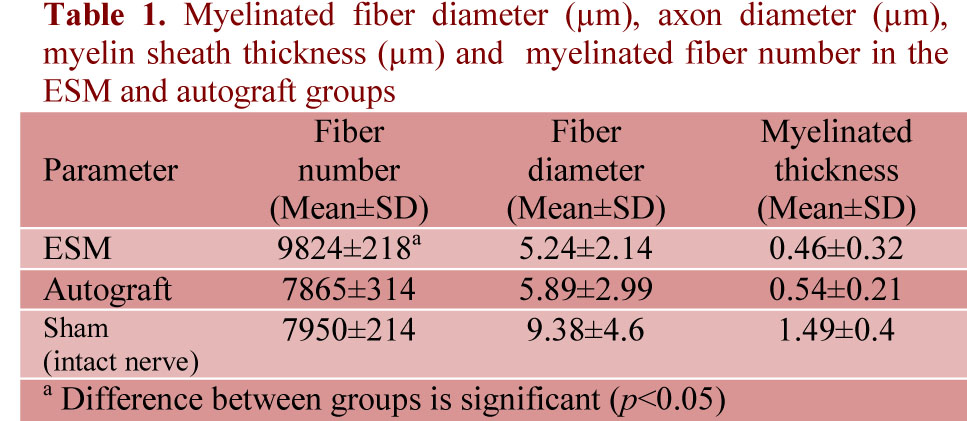
Immunoreactivity to S-100 was extensively observed in the cross section from midpoint of ESM group. The expression of S-100 protein was located mainly in the myelin sheath in ESM and autograft groups. In the ESM group was clearly more positive staining of the myelin sheath-associated protein S-100. The Schwann cell existed around the myelinated axons. In ESM group, the structure of regenerated axons was far more similar to those of normal nerve compared to autograft group (Figure 1).
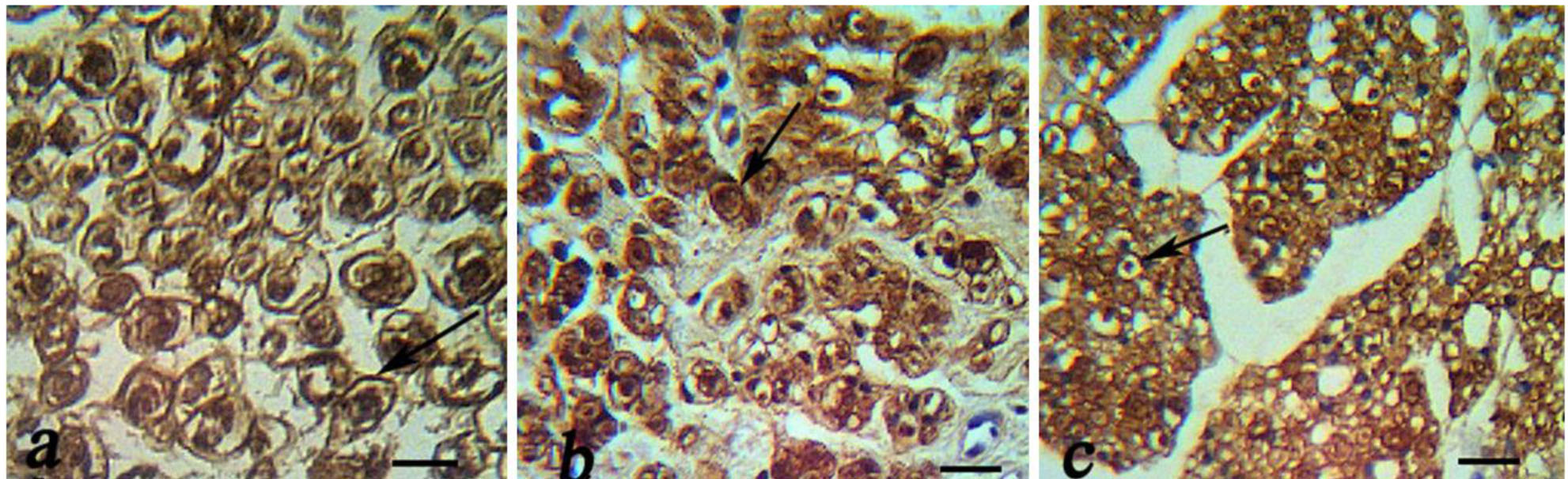
Figure 1: Immunohistochemical analysis of cross sections to the main axis of the regenerated nerve 90 days after surgery from midpoint of normal nerve (a), autograft group (b), and Eggshell membrane (ESM group) (c), and. There was positive staining of the myelin sheath –associated protein S-100 (arrow). There was regenerated nerves fibers containing myelinated axons throughout the tissue, Schwann cells and blood vessels (scale bar 20 µm).
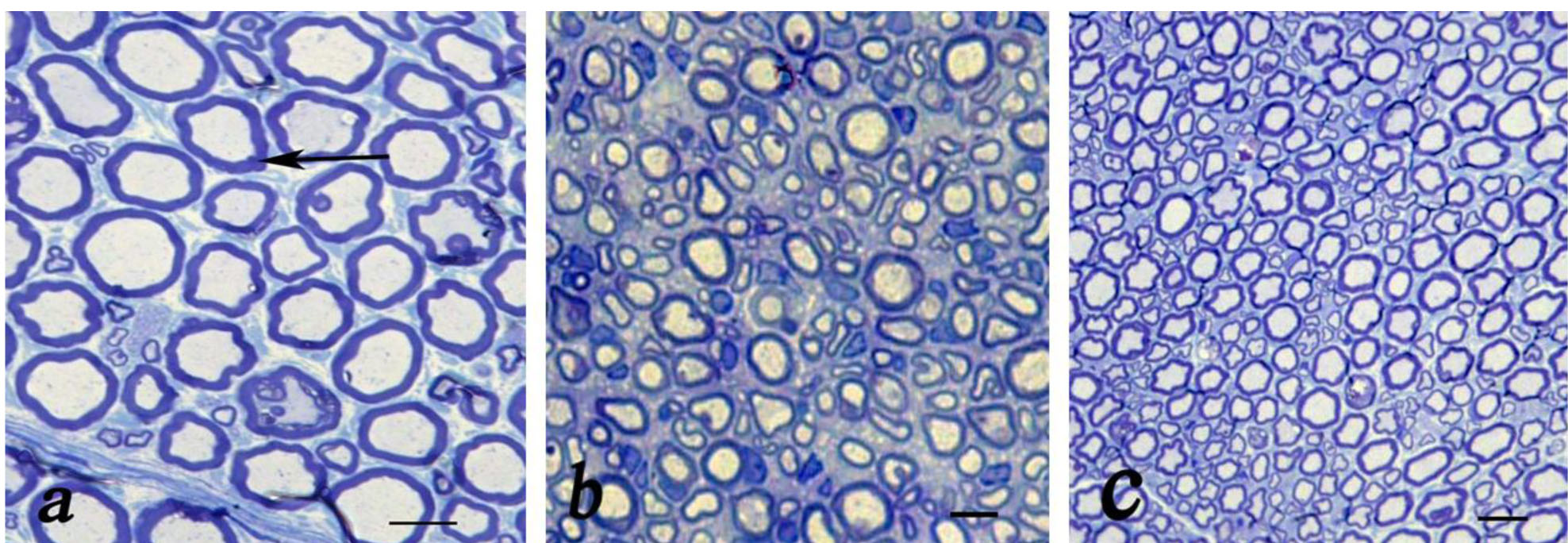
Figure 2: Light microscopic findings of cross sections to the main axis of the regenerated nerve 90 days after surgery from midpoint of normal nerve (a), autograft group (b), and Eggshell (ESM group) (c). Show nerve microfascicles with myelinated axons (arrow) throughout the tissue, Schwann cells, fibrosis and blood vessels (Toluidine blue stain; scale bar 10 µm).
Discussion
The results of this study demonstrate that ESM significantly enhances peripheral nerve regeneration in vivo.
ESM was chosen as NGCs since it has a high content of bioactive components (10) which is easy to be made. In addition, ESM can be obtained in large quantities and is inexpensive, sterilization with ethylene oxide and easily store (20). The chicken ESM contains glycosaminoglycan (approximately 48% hyaluronic acid) (21). Hyaluronic acid has been shown to enhance peripheral nerve regeneration in vitro (22). The high glycine content in all layers of ESM suggests the presence of collagen in the avian eggshell protein matrix (23). The ESM contains collagen type I, V, X (24). The collagen conduit has been shown very efficient as a nerve guidance channel (25).
In the present study, animals were investigated by histological and immunohistochemical assessment. The results demonstrate that both ESM and autograft repairs result in good regeneration. Our previous study showed that ESM promotes walking tract analysis in injured sciatic nerve (19). The walking track analysis clearly demonstrated that there is a direct relationship between individual hind limb muscle function and print measurements (26).
The ESM conduit has a smooth internal surface, and the regenerated cables were round in shape. Round neural cables regenerated in smooth-walled conduits. In the smooth conduit, the nerve cable is centered, free from attachment to the conduit wall and contains blood vessels; also, the axons are grouped in microfascicles and surrounded by an epineurium (27). The ESM conduit has rough external surfaced. The macrophages were found on rough surfaces as opposed to smooth surfaces both in vitro and in vivo (28,29) with evident signs of phagocytosis of the biomaterial. The macrophages can secrete a variety of growth factors that can enhance healing, as well as promoters and regulators of inflammation and tissue degradation (30).
In the present study, the schwann cell existed around the myelinated axons. Presence of schwann cells is necessary for advancement of axons (31), and producing many neurotrophic factors and their receptors (32).
In this study, the average myelin sheath thickness for the autograft group was greater compared to the ESM group, but the difference was not significant. Axon diameter depends on the origin of the axon and maturation of the nerve, which may or may not relate to function. The degree of myelination may relate to the maturity of the axon. However, myelination occurs before the axon reaches end organ, so it may not relate to function. Small axons do not have a thick myelin layer; therefore, the actual myelin thickness may not be an accurate indicator of function (33).
Conclusion
According to the results of this study the ESM conduit is a potential idea nerve conduit material that effectively enhances nerve regeneration, with a mechanism still to be explained. However, further studies are needed to evaluate the efficiency of this ESM conduit in larger gaps, and in association with substances that promote nerve regeneration, such as collagen, laminin, fibronectin, and various growth factors.
Acknowledgments
Funding for this research project was supported by a grant (contract No.932) from Urmia University of Medical Sciences in Urmia, Iran.
Conflict of Interest
The authors have no conflict of interest.
References
- Subramanian A, Krishnan UM, Sethuraman S. Development of Biomaterial Scaffold for Nerve Tissue Engineering: Biomaterial Mediated Neural Regeneration. J Biomed Sci 2009; 16:108-18.
- Seckel BR. Enhancement of Peripheral Nerve Regeneration. Muscle Nerve 1990; 13:785-800.
- Hudson TW, Evans G RD, Schmidt C E. Engineering Strategies for Peripheral Nerve Repair. Clinics In Plastic Surgery 1999; 26 (4): 617-28.
- Johnson EO, Zoubos AB, Soucacos PN. Regeneration and Repair of Peripheral Nerves. Injury 2005; 365:S24-S29.
- Pannunzio ME, Jou I, Long A, Wind TC, Beck G, Balian G. A New Method of Selecting Schwann Cells from Adult Mouse Sciatic Nerve. J Neurosci Methods 2005; 149 (1): 74-81.
- Eppley BL, Snyders RV, Winkelmann TM, Roufa DG. Efficacy of Nerve Growth Factor in Regeneration of the Mandibular Nerve: A Preliminary Report. J Oral Maxillofac Surg 1991; 49: 61-68.
- Johnson EO, Zoubos AB, Soucacos PN. Regeneration and Repair of Peripheral Nerves. Injury 2005; 36:S24-9.
- Dahlin LB. The Biology of Nerve Injury and Repair. JHS 2004; 4 (3): 143-55.
- Torres FG, Troncoso OP, Piaggio F, Higar A. Structure-Property Relationships of a Biopolymer Network: the Eggshell Membrane. Acta Biomater 2010; 6 (9): 3687-3693.
- Cordeiro CM, Hincke MT. Recent Patents on Eggshell: Shell and Membrane Applications. Recent Pat Food Nutr Agric 2011; 3 (1): 1-8.
- Nakano T, Ikawa NI, Ozimek L. Chemical Composition of Chicken Eggshell and Shell Membranes. Poult Sci 2003; 82 (3): 510-14.
- Ruff KJ, Winkler A, Jackson RW, DeVore DP, Ritz BM. Eggshell Membrane in the Treatment of Pain and Stiffness from Osteoarthritis of the Knee: a Randomized, Multicenter, Bouble-Blind, Placebo-Controlled Clinical Study. Clin Rheumatol 2009; 28 (8): 907-14.
- Cordeiro CM, Hincke MT. Recent Patents on Eggshell: Shell and Membrane Applications. Recent Pat Food Nutr Agric 2011; 3 (1): 1-8.
- Tavassoli M. Effect of the Substratum on the Growth of CFU-c in Continuous Marrow Culture. Experientia 1983; 39 (4): 411-12.
- Maeda K, Sasaki Y. An Experience of Hen-Egg Membrane as a Biological Dressing. Burns Incl Therm Inj 1982; 8 (50): 313-16.
- 16-Yi F, Guo Z, Zhang L, Yu J, Li Q. Soluble Eggshell Membrane Protein: Preparation, Characterization and Biocompatibility. Biomaterials 2004; 25: 4591-99.
- Jia J, Duan YY, Yu J, Lu JW. Preparation and Immobilization of Soluble Eggshell Membrane Protein on the Electrospun Nanofibers to Enhance Cell Adhesion and Growth. J Biomed Mater Res 2008; 86 (2): 346-73.
- Arias JI, Gonzalez A, Fernandez MS, Gonzalez C, Saez D, Arias JL. Eggshell Membrane as a Biodegradable Bone Regeneration Inhibitor. J Tissue Regen Med 2008; 2 (4): 228-35.
- Farjah Gh, Heshmatian B, Karimipour M, Saberi A. Using Eggshell Membrane as Nerve Guide Channels in Peripheral Nerve Regeneration. Iran J Basic Med Sci 2013; 16: 901- 5.
- Leighton J, Mansukhani S, Estes L. Decalcified Eggshell Membrane, a Supporting Substrate for Electron Microscopic Cross Sections of Monolayers of Epithelial Cell Line MDCK. IN Vitro 1971; 6 (4): 251-2.
- Nakano T, Ikawa N, Ozimek L. Extraction of Glycosaminoglycans from Chicken Eggshell Poultry. Science 2001; 80: 681-4.
- Wang KK, Nemeth IR, Seckel BR, Seckel BR. Hyaluronic Acid Enhances Peripheral Nerve Regeneration In Vivo. Microsurgery 1998; 18(4):270-5.
- Miksik L, Eckhadt A, Sedlakova P, Mikulikova K. Proteins of Insoluble Matrix of Avian (gallus gallus) Eggshell. Connect Tissue res 2007; 48 (1): 1-8.
- Arias JL, Carrino DA, Fernandez MS, Rodriguez JP, Dennis JE, Caplan AL. Partial Biochemical and Immunochemical Characterization of Avian Eggshell Extracellular Matrices. Arch Biochem Biophys 1992; 298 (1): 293-302.
- Kitahara A K, Suzuki Y, Qi P, Nishimura Y, Suzuki K, Kiyotani T, et al. Evaluation of Collagen Nerve Guide in Facial Nerve Regeneration. J Artif Organs 1998; 1: 22-7.
- Reynolds JL, Urbanchek MS, Asato H, Kuzon WM JR. Deletion of Individual Muscles Alters Rat Walking-Track Parameters. J Reconstr Microsurg 1996; 12 (7): 461-66.
- Aebischer P, Guenard V, Valentini RF. The Morphology of Regenerating Peripheral Nerves Is Modulated by the Surface Microgeometry of Polymeric Guidance Channels. Brain Res 1990; 531:211-18.
- Rich A, Harris AK. Anomalous Preferences of Cultured Macrophages for Hydrophobic and Roughened Substrata. J Cell Sci 1981;50: 1-7.
- Salthouse TN. Some Aspects of Macrophage Behavior at the Implant Interface. J Biomed Mater Res 1984; 18 (4): 395-401.
- Chamberlain LJ, Yannas IV, Arrizabalaga A, Hsu HP, Norregaard TV, Spector M. Early Peripheral Nerve Healing in Collagen and Silicone Conduit Implants: Myofibroblasts and the Cellular Response. Biomaterials 1998; 19 (15): 1393-403.
- Lundborg G. Nerve Regeneration and Repair. A review. Acta Orthop Scand 1987; 58 (2): 145-69.
- Mirsky R, Jessen KR. Schwann Cell Development, Differentiation and Myelination. Curr Opin Neurobiol 1996; 6 (1): 89-96.
- Kanaya F, Firrell JC, Breidenbach WC. Sciatic Function Index, Nerve Conduction Tests, Muscle Contraction, and Axon Morphometry as Indicators of Regeneration. Plast Reconstr Surg 1996; 98 (7): 1264-74.
Received: 2016/03/6 | Accepted: 2016/03/6 | Published: 2016/03/6
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |




