Wed, Apr 24, 2024
Volume 7, Issue 2 (Spring 2021)
Caspian J Neurol Sci 2021, 7(2): 118-131 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Haghani Dogahe M, Feizkhah A, Seddighi S, Kiani P, Shafaei F. Application of Magnetic Resonance Spectroscopy in Neurocognitive Assessment After Head Injury: A Systematic Review. Caspian J Neurol Sci 2021; 7 (2) :118-131
URL: http://cjns.gums.ac.ir/article-1-420-en.html
URL: http://cjns.gums.ac.ir/article-1-420-en.html
1- Department of Neurosurgery, Guilan University of Medical Sciences, Rasht, Iran.
2- Burn and Regenerative Medicine Research Center, Guilan University of Medical Sciences, Rasht, Iran.
3- Medical Futurology Student Scientific Association, Burn and Regenerative Research Center, Guilan University of Medical Sciences, Rasht, Iran.
4- Department of Neuroscience and Addiction Studies, School of Advanced Technologies in Medicine, Tehran, Iran.
5- Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
2- Burn and Regenerative Medicine Research Center, Guilan University of Medical Sciences, Rasht, Iran.
3- Medical Futurology Student Scientific Association, Burn and Regenerative Research Center, Guilan University of Medical Sciences, Rasht, Iran.
4- Department of Neuroscience and Addiction Studies, School of Advanced Technologies in Medicine, Tehran, Iran.
5- Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
Keywords: Magnetic resonance spectroscopy, Brain injuries, Brain concussion, Neurocognitive disorders
Full-Text [PDF 2039 kb]
(526 Downloads)
| Abstract (HTML) (1516 Views)
Full-Text: (646 Views)
Introduction
raumatic Brain Injury (TBI) is a major area of interest within the field of Magnetic Resonance Spectroscopy (MRS). Neurocognitive disorders have been an essential concept in the study of brain trauma [1]. Because TBI is prevalent, Post-Traumatic Neurocognitive Disorders (PTND) are becoming increasingly important [2].
Detecting these pathological changes before affecting patients’ life can be essential for a wide range of solutions. Specifically, having a reliable tool can play a vital role in monitoring the therapeutic interventions [1, 2, 3, 4, 5].
Several studies showed the involvement of different brain structures in the pathogenesis of traumatic brain injuries [6]. On the other hand, the concepts of probable underlying biochemical etiology of neurocognitive disorders after brain trauma raising the importance of neurochemistry evaluations in this area [1, 4]. Trauma is a well-known condition that has a considerable impact on metabolite alterations [7]. Determining the role of metabolite alterations in PTNDs is vital for expanding the frontiers of neurocognitive disorders [2, 4].
Advanced neuroimaging provides non-invasive techniques to reach a more helpful concept of concussion [2, 4]. One of the most potent modalities is MRS. In the last few decades, there has been a surge of interest in the importance of MRS as a modality for detecting metabolite alterations after TBI [1]. Magnetic resonance spectroscopy is a non-invasive neuroimaging technique that measures metabolic levels based on chemical alterations, i.e., frequency deviations from a standard reference in selected regions of interest in a tissue. As it is a quantitative technique, it can be used as a diagnostic and predictor modality in various diseases [8, 9]. MRS can be applied in single-voxel or multi-voxel settings. Multi-voxel MRS is also referred to as Magnetic Resonance Spectroscopy Imaging (MRSI) or Chemical Shift Imaging (CSI) [10].
Various known nuclei are available to use in medical and pharmacological research, such as proton (1H), fluorine (19F), phosphate (31P), carbon (13C), and sodium (23Na). As water molecules are abundant molecules in the human body, H-MRS (proton magnetic resonance spectroscopy). Thus, in routine MRI scanners available in clinical studies (1.5 T and 3 T), H-MRS is most commonly used [8].
The number of detected metabolites depends on the magnetic field strength of the MRI scanner and acquisition sequence [11]. N-acetyl aspartate (NAA), creatine (Cr), choline (Cho), and Myo-inosito (MI) are the most important metabolites of the brain that are commonly detected in MRS studies. The peak of each metabolite arises from several compositions. Stronger scanners can detect extra-metabolites such as glutamate, glutamine, glutathione, gamma-aminobutyric acid (GABA), and lactate [12, 13, 14]. Some studies are pointing to the neglected relationship between neuro-metabolites alterations and PTND. This paper aims to assess these relationships.
Materials and Methods
Search Strategy
This study comprehensively reviews the data to trace the relationship between neuro-metabolite alterations and PTND. We followed preferred reporting items for systematic review and meta-analysis (PRISMA) (www.prisma-statement.org) [15] (Figure 1). The systematic search was performed in Medline and Embase databases. Unpublished articles were not included. Notably, the search was performed from the first edition of electronic databases until March 21, 2021.
Keywords
The combinations of the following keywords were used in the search strategy: (magnetic resonance spectroscopy (title/abstract) AND ((head trauma) OR (head injury) OR (head injuries) OR (Traumatic Brain Injury) OR (traumatic brain injuries) OR (concussion) (title/abstract).
Exclusion and inclusion criteria
When the search was completed, citation titles and abstracts were reviewed. Non-English articles, unrelated articles, topic/narrative/systematic reviews, and irrelevant studies were excluded. Also, MRS studies without data for PTND were excluded. We included all articles that report any relation (association, correlation, or regression model) between MRS data and PTND.
Quality assessment
Quality assessment performed using a modified version of the ROBINS-I (risk of bias in non-randomised studies of interventions) tool to classify studies as low, high, or unclear risk judgments based on evaluation of confounding, selection, classification of intervention(s), missing data, and the measure of outcome(s) [16, 17].
Data extraction
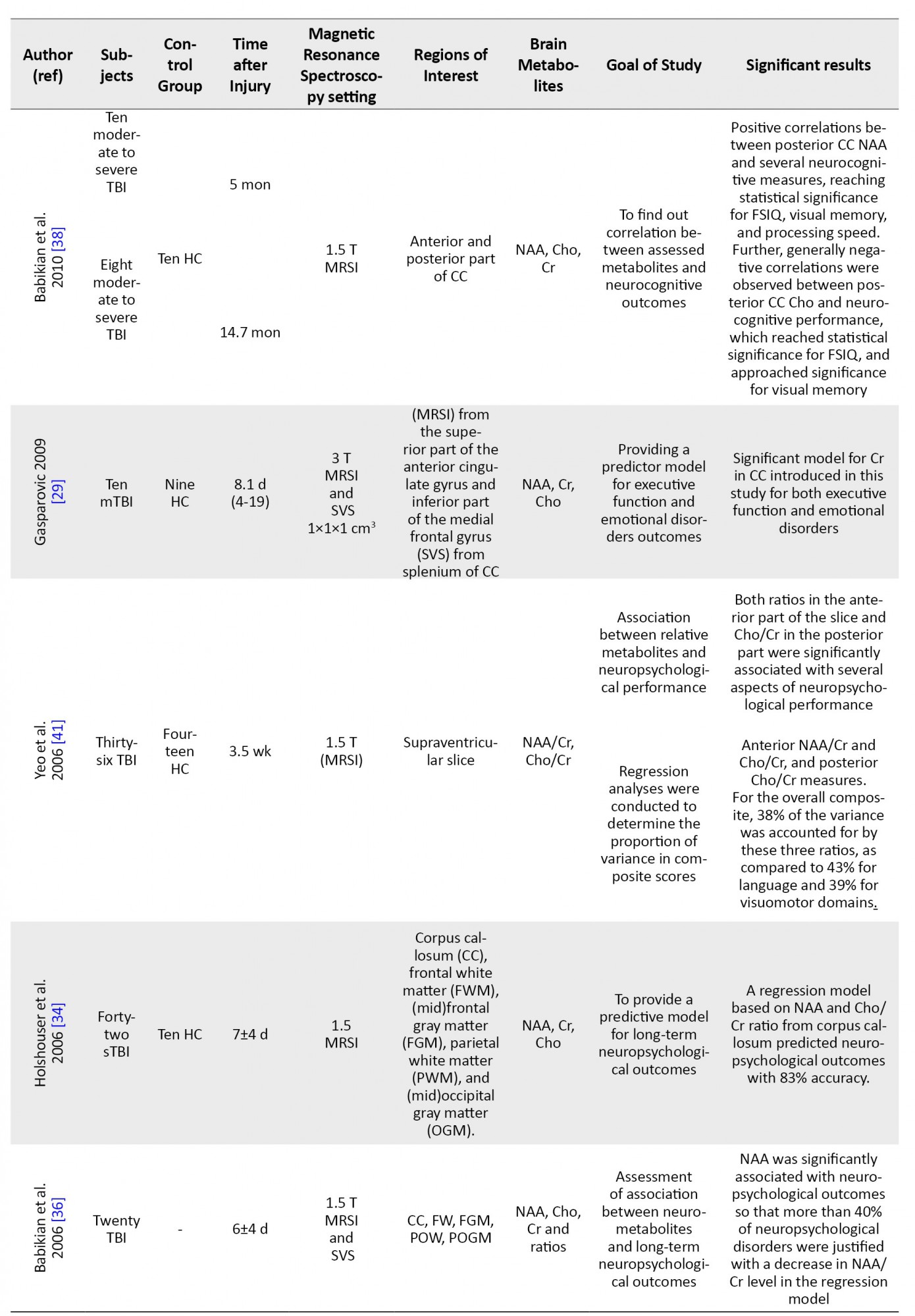
After removing duplicates, all articles were screened independently in a blinded standardized manner. We collected groups involved in studies, duration between injury and imaging, MRS settings, regions and metabolites of interest, and bold findings of each study.
Results
The literature search yielded 22 relevant articles (Figure 1). The studies are summarized in Table 1.
.jpg)


Although some studies are designed due to the availability of MRS scanners, both MRS and MRSI have advantages and disadvantages. Some expertise preferred to use both voxels in different pathologies.
While MRSI can be performed in 2D or 3D and detects a broader area of the brain, single voxel spectroscopy (SVS) provides higher quality spectra. Furthermore, to apply SVS, a region of interest needs to be defined. Hence, when the researchers want to detect a selective structural region, SVS is preferable [8, 11, 18].
According the results, Anterior Cingulate Cortex (ACC) [19, 20], posterior cingulate cortex [20, 21], frontal [22] and parietal white matter [20, 21, 23], prefrontal cortex [24] dorsolateral prefrontal cortex (DLPFC) [25, 26, 27], corpus callosum [28, 29], occipital gray matter [23, 30, 31], parieto-occipital white matter [31], basal ganglia [32], and mid-temporal cortex [32] contribute significantly in neurocognitive functions. We believe that the ACC and prefrontal cortex are important structural parts of the brain with a significant effect on neuro-cognitive pathways such as Papez and emotion circuits [33].
MRSI studies are more focused on the supraventricular area to cover corpus callosum level [34, 35, 36, 37, 38], thalamus, centrum semiovale [2], ant-post commissure axis, ACC [29], frontal gyri, and superior longitudinal fasciculus.
Interested metabolites were N-acetylaspartate, choline (Cho), creatine (Cr), and MI in the majority of studies. Increasing interest for extra-metabolites such as glutamine, glutamate, and glutathione was seen over time.
Neurocognitive Disorders and Evaluation Tools
Several tests are used to evaluate neurocognitive function after TBI. Diagnostic and Statistical Manual of Mental Disorders criteria can be assessed in the concussed patient diagnosis of post-concussion syndrome. To quantify Post-Concussion Syndrome (PCS) grading, Rivermead Post-Concussion Syndrome Questionnaire (RPQ) is a valid questionnaire. The RPQ asks participants to rate a series of common symptoms following TBI on a 5-point Likert-type scale from 0 to 4 (King et al., 1995) [35]. Sours et al. reported that the Cho/Cr ratio in the thalamus of mTBI (mild Traumatic Brain Injury) patients who self-reported sensory symptoms on the RPQ is significantly higher compared to mTBI patients who did not report sensory symptoms at the acute time point (after controlling the age influence) [35].
Mini-mental state examination and military acute concussion evaluation are other questionnaires available to assess general mental functions [35].
However, these questionnaires provide an essential view of the patients’ quality of life; behavioral tests. Full-scale IQ (FSIQ) score, Wechsler abbreviated intelligence scale, California verbal learning test–second edition or its children’s version, Wechsler memory scale–third edition, and children’s memory scale are some tests used to assess behavioral functions [38]. In addition, there are many computerized tests to assess cognitive situations such as backward, digit span, Stroop test A, and trail making test (TMT)-B time.
In this regard, Sivak et al. reported positive correlations between NAA level and cognitive tests (backward, digit span, Stroop test A, TMT-B time). Also, NAA/Cr was associated with Stoop test A and the total score of digit span [27]. Finally, some studies used the Glasgow Outcome Scale (GOS) to evaluate the outcomes of traumatic patients. While GOS is more accurate for moderate to severe injuries, few studies reported GOS for mTBI.
Govindaraju et al. reported that metabolite ratios were not significantly correlated with GCS score at admission or six months after injury, although they were weakly correlated with GOS score at discharge. There was some evidence of a weak correlation between NAA/Cho and GOS score on discharge approaching statistical significance. However, this GOS does not seem to be an appropriate criterion in mTBI.
Predictive value of MRS and Post-traumatic Neurocognitive Disorders
Few studies have reported on the prognostic value of MRS in TBI using regression models. Holshouster et al. found that neuro-metabolite changes in corpus callosum can predict neurocognitive outcomes with 83% accuracy. The majority of these outcomes are expressed by NAA/Cr and Cho/Cr ratios.
In this regard, Gasparovic et al. introduced a regression model based on Cr in corpus callosum for executive functions outcome. He also reported that this model was significant for emotional disorders [27]. Despite these results, further studies are needed to find the most accurate MRS result for outcomes in TBI models.
Discussion
Returning to the question posed at the beginning of this study, it is now possible to state that magnetic resonance spectroscopy can detect dynamic biomarkers of neuronal dysfunction at an earlier stage of disease progression.
It seems likely that MRS is a useful tool for diagnosis and therapeutic monitoring approaches [42]. It can be used in two ways: Single-Voxel Spectroscopy (SVS) and Magnetic Resonance Spectroscopy Imaging (MRSI) or Chemical Shift Imaging (CSI).
Biochemistry: Background in TBI
N-acetylaspartate (NAA) is an acetyl-amino acid that resonates at 2.01 ppm in the H-MRS. NAA originates from mitochondria and reflects neuronal integrity and viability [43]. Most research supports a diffuse nature of NAA decrease after traumatic brain injuries [26، 27, 31, 34, 37, 44, 45, 46, 47, 48, 49, 50, 51, 52]. Nevertheless, despite several similar results, some studies did not find any changes in NAA after TBI [53, 54]. We believe that the main reason for these results was the prolonged interval between injury and imaging [55, 56]. These data do not role out hyperacute metabolite alterations and the value of early metabolite alterations in clinical evaluations [53].
Choline (Cho) or total choline includes free choline, glycerol-phosphocholine, and phosphocholine. It is observed as a prominent singlet at 3.2 ppm in the H-MRS [11, 13]. However, the range of Cho changes is too tiny. Such changes may associate with changes in membrane turnover or brain injuries [13]. Regarding the literature, the increase in Cho level in the TBI group compared with the healthy group is due to tissue breakdown [9, 62, 63]; Recently, Babikian et al. argued that lower lobar Cho levels in the chronic stage of TBI were correlated with declined Interhemispheric Transfer Time (IHTT) in moderate to severe TBI subgroups [69]. Nevertheless, probably Cho levels would be higher in more severe TBI cases, compared to a healthy population; however, the lower level of Cho in patients with more severe neurocognitive outcomes, compared to patients with better outcomes may be attributed to decreased membrane turnover in the setting of permanent cellular damage [28, 55].
Creatine (Cr) is an indirect intermediator of the energy used as a marker for the total stored energy of the cell [57, 58]. Cr peak resonates at 3.03 ppm [13]. A decrease in Cr in the traumatic infarction area supports dropping Cr level when the cell is dead [59]. Yeo et al. found a significant negative correlation between Cr and days after injury, suggesting that damaged cell repair occurs using cell storage energy [53]. Of note, an interval of 3 to 5 months for complete biochemically repairing of the injured cells suggests that this interval may associate with Cr level base as a marker for cellular energy [29].
The MI is another metabolite that can be detected using short echo time spectroscopy [31]. MI, known as an osmolyte, increases parallel to activation of microgliosis following TBI. Contrary to NAA, Cr, and Cho, which have a trend toward average over time, it is believed that MI level increases [6]. Still, there is no evidence of significant MI changes on the day of brain injury. Since MI is a gliotic marker, an increase in MI is expected in subacute and chronic TBI stages [6]. It has to be mentioned that some extra-metabolites are less common to calculate by MRS due to their very low concentration in the brain. Glutamate, glutamine, glutathione, GABA, and lactate are important extra metabolites that MRS can detect.
Glutamate (Glu) is an excitatory neurotransmitter that converts to glutamine (Gln) within astrocytes. Because most clinical MRI scanners are 1.5 T, Glu and Gln are usually reported in combination due to their overlapping resonances [60]. Elevated Glx and Glu following TBI are supposed to happen due to excitotoxicity [29]
Glutathione is a tripeptide thiol that acts as an oxidation-reduction cofactor in enzyme reactions [61].
γ-Aminobutyric acid (GABA) is the main inhibitory neurotransmitter in the human cortex. Literature indicates that GABA may play a potential role in cognitive dysfunctions following TBI [62]. A few previous translational studies have suggested that neurotransmitters, including acetylcholine, glutamate, dopamine, serotonin, and GABA, may potentially be biomarkers of cognitive dysfunctions in TBI [40]. Lactate occupies a unique position in energy metabolism. It is believed that the presentation of lactate in MRS suggests a poorer prognosis [63].
Absolute Neuro-Metabolites and Post-Traumatic Neurocognitive Disorders
N-Acetylaspartate (NAA) is the commonest neuro-metabolite assessed by the MRS. The majority of studies found a lower level of NAA in traumatic patients compared with control groups in various parts of the brain [32]. Research supports a strong relationship between decreased NAA level and neurocognitive outcomes [22, 36, 40, 64, 65, 66]. According to a study performed by Babikian et al., NAA explains over 40% of the variance in long-term outcomes of the cognitive functions [36]. MacMaster et al. found a significant association between decreased NAA in the left dorsolateral prefrontal cortex (DLPFC) and emotional symptoms in youth with a concussion in a recent study. In addition, a significant negative correlation exists between NAA level and emotional stress degree [26]. Previously, Sivak et al. reported a significant correlation between NAA in left DLPFC and computerized cognitive tests (backward digit span, Stroop test A, TMT-B time) [27].
However, most studies did not report a significant correlation between neuro-metabolites and computerized tests. Some authors reported a significant negative correlation between NAA and computerized cognitive tests. Babikian et al. noted a positive correlation between NAA in the posterior part of the corpus callosum and several neurocognitive measures, reaching statistical significance for FSIQ, visual memory, and processing speed [38]. Although longitudinal studies support that increased NAA level towards a normal value after 30 days of injury is related to better long-term outcomes [67].
Choline (Cho) increase after TBI has been reported by some studies [37]. Some are reported the association between the increase mentioned above with insignificant outcomes [23, 64, 68]. In contrast, Parry et al. found that lower levels of Cho displayed reduced performances on neurocognitive tests [23]. Babikian et al. evaluated the correlation between single-voxel spectroscopy (SVS) data from the anterior and posterior part of the corpus callosum and neurocognitive performance. In this regard, the authors reported a positive correlation in the anterior part for response time, while in the posterior part, they concluded that this metabolite was statistically significant for FSIQ and approached significance for visual memory [38].
In a more recent study, Babikian et al. showed that lower lobar Cho levels in the chronic stage of TBI were correlated with lower interhemispheric transfer time (IHTT) in moderate to severe TBI subgroups [69].
Nevertheless, We believe that probably Cho level would be higher in more severe TBI than in a healthy population; but the lower level of Cho in the patients with more severe neurocognitive outcomes comparing patients with better outcomes may be due to decreased membrane turnover in the setting of permanent cellular damage [28, 55].
Few specialists believe that the creatine (Cr) level is stable in different situations [38], while some studies report that Cr can be changed significantly due to different situations [2, 29]. Gasparovic et al. introduced a significant regression model to predict emotional disorders based on Cr level in the splenium of the CC and white matter of the cingulate gyrus [29]. In addition, George et al. and Babikian et al. found a significant positive correlation between the acute phase of Cr level and neurocognitive outcomes in traumatic brain injuries [2, 38].
Current studies showed more interest in detecting MI in the sub-acute and chronic phases after traumatic brain injuries [20, 21, 31]. In this context, Ashwal et al. found a significant association between higher MI levels in occipital gray matter and poorer outcomes 6-12 months after injury [31]. Recently Alosco et al. published a positive correlation between MI from anterior cingulate cortex and behavioral/mood symptoms in patients with repetitive impact injury history comparing non-exposed control groups [20]. A study performed by Gardner et al. showed that in retired athletes, significant positive correlations between DASS (depression anxiety stress scale) and grey matter MI suggest that increasing MI levels are associated with worse anxiety symptoms in the posterior cingulate cortex [21]. In addition, the authors also reported a significant correlation between RPQ score and MI level in this area [21].
There are some neuro-metabolites which MRS does not usually detect. A few studies reported significant associations between GLx from occipital gray matter and parietal white matter [23]. Shutter et al. indicate that Cho and GLx have 94% accuracy in predicting long-term traumatic brain injuries [23]. Recently, Sheth et al. reported a lower glutamine level in the dorsal anterior cingulate cortex [19]. Besides, few studies have demonstrated a positive correlation between glutamate, glutathione, and behavioral/mood symptoms [20, 69]. Gardner et al. designed a study to examine if brain neuro-metabolite concentrations are associated with neurocognitive disorders in retired rugby league players who had a history of numerous self-reported concussions. The authors performed MRS in the posterior cingulate cortex and parietal white matter using 3 T single-voxel spectroscopy. The results showed that DASS anxiety scores were positively correlated with grey matter glutamate and Glx. Also, Rivermead post-concussion syndrome scores were positively correlated with grey matter glutamate. In addition, BESS scores were negatively correlated with grey matter glutathione and white matter glutathione [21].
Another interested extra-metabolite is GABA. Some studies support the role of GABA in post-traumatic stress disorders [19]. In a recent study, Kim et al. showed that the GABA level in the prefrontal cortex was correlated with memory performance in the Boxers but not in attention performance [24]. Lastly, some studies report that the presence of high lactate in patients with traumatic brain injuries had weaker association outcomes [30, 37].
Relative Neuro-Metabolites and Post-Traumatic Neurocognitive Disorders
Some experts believe that the relative value of metabolites is more reliable than the absolute value of metabolites [58]. Although there are some controversies, some authors believe that the Cr level remains stable in pathologic situations. Thus, most studies have used Cr as a base for relative measures [2, 27, 35, 41].
Sivak et al. found significant correlations between NAA/Cr in the right DLPFC and neurocognitive tests (total digit span and Stroop test) [27]. George et al. showed that NAA/Cr ratio in the thalamus is significantly associated with cognitive tests [2]. Likewise, many studies reported an association between reductions in NAA/Cr and poor long-term neurocognitive performance [30, 41]. NAA/Cho is another ratio that has been reported as a valuable relationship with poor long-term neurological outcomes [30, 32].
Cho/Cr is another essential ratio in the setting of TBI. An increasing trend of Cho/Cr in DLPFC in patients with PCS is reported by Dean et al. [25]. Another study by Dean et al. showed an increase in Cho/Cr ratio is correlated with the Rivermead Post-Concussion Syndrome Questionnaire Score (RPQS) [71]. Nevertheless, some studies report that Cho/Cr ratio is higher in traumatic groups with poorer outcomes [30].
A chemical shift imaging study performed by Yeo et al. on Cho/Cr ratios from the frontal lobe to the posterior parietal lobes and sampling from both white and gray matter [41]. Thus, the relationship was confirmed in other parts of the brain, such as the thalamus [35].
Conclusion
We found that MRS data can provide essential data for clinicians to predict neurocognitive outcomes following traumatic brain injuries. Neuro-metabolite ratios provide a broader information to evaluate cellular integrity in brain tissue. Further studies are needed to prepare predictive models based on MRS data for post-traumatic neurocognitive disorders.
Ethical Considerations
Compliance with ethical guidelines
All study procedures were done in compliance with the ethical guidelines of the 2013 version of the Declaration of Helsinki.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and Supervision: Mohammad Haghani Dogahe; Methodology: Mohammad Haghani Dogahe and Alireza Feizkhah; Investigation: Mohammad Haghani Dogahe, Alireza Feizkhah, and Sara Seddighi; Writing the original draft, review, and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank Dr. Mohammadreza Mobayen (Burn and Regenerative Medicine Research Center, Guilan University of Medical Sciences, Rasht City, Iran) for his expert advice and encouragement throughout this project and for providing a suitable place for researchers.
References
raumatic Brain Injury (TBI) is a major area of interest within the field of Magnetic Resonance Spectroscopy (MRS). Neurocognitive disorders have been an essential concept in the study of brain trauma [1]. Because TBI is prevalent, Post-Traumatic Neurocognitive Disorders (PTND) are becoming increasingly important [2].
Detecting these pathological changes before affecting patients’ life can be essential for a wide range of solutions. Specifically, having a reliable tool can play a vital role in monitoring the therapeutic interventions [1, 2, 3, 4, 5].
Several studies showed the involvement of different brain structures in the pathogenesis of traumatic brain injuries [6]. On the other hand, the concepts of probable underlying biochemical etiology of neurocognitive disorders after brain trauma raising the importance of neurochemistry evaluations in this area [1, 4]. Trauma is a well-known condition that has a considerable impact on metabolite alterations [7]. Determining the role of metabolite alterations in PTNDs is vital for expanding the frontiers of neurocognitive disorders [2, 4].
Advanced neuroimaging provides non-invasive techniques to reach a more helpful concept of concussion [2, 4]. One of the most potent modalities is MRS. In the last few decades, there has been a surge of interest in the importance of MRS as a modality for detecting metabolite alterations after TBI [1]. Magnetic resonance spectroscopy is a non-invasive neuroimaging technique that measures metabolic levels based on chemical alterations, i.e., frequency deviations from a standard reference in selected regions of interest in a tissue. As it is a quantitative technique, it can be used as a diagnostic and predictor modality in various diseases [8, 9]. MRS can be applied in single-voxel or multi-voxel settings. Multi-voxel MRS is also referred to as Magnetic Resonance Spectroscopy Imaging (MRSI) or Chemical Shift Imaging (CSI) [10].
Various known nuclei are available to use in medical and pharmacological research, such as proton (1H), fluorine (19F), phosphate (31P), carbon (13C), and sodium (23Na). As water molecules are abundant molecules in the human body, H-MRS (proton magnetic resonance spectroscopy). Thus, in routine MRI scanners available in clinical studies (1.5 T and 3 T), H-MRS is most commonly used [8].
The number of detected metabolites depends on the magnetic field strength of the MRI scanner and acquisition sequence [11]. N-acetyl aspartate (NAA), creatine (Cr), choline (Cho), and Myo-inosito (MI) are the most important metabolites of the brain that are commonly detected in MRS studies. The peak of each metabolite arises from several compositions. Stronger scanners can detect extra-metabolites such as glutamate, glutamine, glutathione, gamma-aminobutyric acid (GABA), and lactate [12, 13, 14]. Some studies are pointing to the neglected relationship between neuro-metabolites alterations and PTND. This paper aims to assess these relationships.
Materials and Methods
Search Strategy
This study comprehensively reviews the data to trace the relationship between neuro-metabolite alterations and PTND. We followed preferred reporting items for systematic review and meta-analysis (PRISMA) (www.prisma-statement.org) [15] (Figure 1). The systematic search was performed in Medline and Embase databases. Unpublished articles were not included. Notably, the search was performed from the first edition of electronic databases until March 21, 2021.
Keywords
The combinations of the following keywords were used in the search strategy: (magnetic resonance spectroscopy (title/abstract) AND ((head trauma) OR (head injury) OR (head injuries) OR (Traumatic Brain Injury) OR (traumatic brain injuries) OR (concussion) (title/abstract).
Exclusion and inclusion criteria
When the search was completed, citation titles and abstracts were reviewed. Non-English articles, unrelated articles, topic/narrative/systematic reviews, and irrelevant studies were excluded. Also, MRS studies without data for PTND were excluded. We included all articles that report any relation (association, correlation, or regression model) between MRS data and PTND.
Quality assessment
Quality assessment performed using a modified version of the ROBINS-I (risk of bias in non-randomised studies of interventions) tool to classify studies as low, high, or unclear risk judgments based on evaluation of confounding, selection, classification of intervention(s), missing data, and the measure of outcome(s) [16, 17].
Data extraction
After removing duplicates, all articles were screened independently in a blinded standardized manner. We collected groups involved in studies, duration between injury and imaging, MRS settings, regions and metabolites of interest, and bold findings of each study.
Results
The literature search yielded 22 relevant articles (Figure 1). The studies are summarized in Table 1.
.jpg)



Although some studies are designed due to the availability of MRS scanners, both MRS and MRSI have advantages and disadvantages. Some expertise preferred to use both voxels in different pathologies.
While MRSI can be performed in 2D or 3D and detects a broader area of the brain, single voxel spectroscopy (SVS) provides higher quality spectra. Furthermore, to apply SVS, a region of interest needs to be defined. Hence, when the researchers want to detect a selective structural region, SVS is preferable [8, 11, 18].
According the results, Anterior Cingulate Cortex (ACC) [19, 20], posterior cingulate cortex [20, 21], frontal [22] and parietal white matter [20, 21, 23], prefrontal cortex [24] dorsolateral prefrontal cortex (DLPFC) [25, 26, 27], corpus callosum [28, 29], occipital gray matter [23, 30, 31], parieto-occipital white matter [31], basal ganglia [32], and mid-temporal cortex [32] contribute significantly in neurocognitive functions. We believe that the ACC and prefrontal cortex are important structural parts of the brain with a significant effect on neuro-cognitive pathways such as Papez and emotion circuits [33].
MRSI studies are more focused on the supraventricular area to cover corpus callosum level [34, 35, 36, 37, 38], thalamus, centrum semiovale [2], ant-post commissure axis, ACC [29], frontal gyri, and superior longitudinal fasciculus.
Interested metabolites were N-acetylaspartate, choline (Cho), creatine (Cr), and MI in the majority of studies. Increasing interest for extra-metabolites such as glutamine, glutamate, and glutathione was seen over time.
Neurocognitive Disorders and Evaluation Tools
Several tests are used to evaluate neurocognitive function after TBI. Diagnostic and Statistical Manual of Mental Disorders criteria can be assessed in the concussed patient diagnosis of post-concussion syndrome. To quantify Post-Concussion Syndrome (PCS) grading, Rivermead Post-Concussion Syndrome Questionnaire (RPQ) is a valid questionnaire. The RPQ asks participants to rate a series of common symptoms following TBI on a 5-point Likert-type scale from 0 to 4 (King et al., 1995) [35]. Sours et al. reported that the Cho/Cr ratio in the thalamus of mTBI (mild Traumatic Brain Injury) patients who self-reported sensory symptoms on the RPQ is significantly higher compared to mTBI patients who did not report sensory symptoms at the acute time point (after controlling the age influence) [35].
Mini-mental state examination and military acute concussion evaluation are other questionnaires available to assess general mental functions [35].
However, these questionnaires provide an essential view of the patients’ quality of life; behavioral tests. Full-scale IQ (FSIQ) score, Wechsler abbreviated intelligence scale, California verbal learning test–second edition or its children’s version, Wechsler memory scale–third edition, and children’s memory scale are some tests used to assess behavioral functions [38]. In addition, there are many computerized tests to assess cognitive situations such as backward, digit span, Stroop test A, and trail making test (TMT)-B time.
In this regard, Sivak et al. reported positive correlations between NAA level and cognitive tests (backward, digit span, Stroop test A, TMT-B time). Also, NAA/Cr was associated with Stoop test A and the total score of digit span [27]. Finally, some studies used the Glasgow Outcome Scale (GOS) to evaluate the outcomes of traumatic patients. While GOS is more accurate for moderate to severe injuries, few studies reported GOS for mTBI.
Govindaraju et al. reported that metabolite ratios were not significantly correlated with GCS score at admission or six months after injury, although they were weakly correlated with GOS score at discharge. There was some evidence of a weak correlation between NAA/Cho and GOS score on discharge approaching statistical significance. However, this GOS does not seem to be an appropriate criterion in mTBI.
Predictive value of MRS and Post-traumatic Neurocognitive Disorders
Few studies have reported on the prognostic value of MRS in TBI using regression models. Holshouster et al. found that neuro-metabolite changes in corpus callosum can predict neurocognitive outcomes with 83% accuracy. The majority of these outcomes are expressed by NAA/Cr and Cho/Cr ratios.
In this regard, Gasparovic et al. introduced a regression model based on Cr in corpus callosum for executive functions outcome. He also reported that this model was significant for emotional disorders [27]. Despite these results, further studies are needed to find the most accurate MRS result for outcomes in TBI models.
Discussion
Returning to the question posed at the beginning of this study, it is now possible to state that magnetic resonance spectroscopy can detect dynamic biomarkers of neuronal dysfunction at an earlier stage of disease progression.
It seems likely that MRS is a useful tool for diagnosis and therapeutic monitoring approaches [42]. It can be used in two ways: Single-Voxel Spectroscopy (SVS) and Magnetic Resonance Spectroscopy Imaging (MRSI) or Chemical Shift Imaging (CSI).
Biochemistry: Background in TBI
N-acetylaspartate (NAA) is an acetyl-amino acid that resonates at 2.01 ppm in the H-MRS. NAA originates from mitochondria and reflects neuronal integrity and viability [43]. Most research supports a diffuse nature of NAA decrease after traumatic brain injuries [26، 27, 31, 34, 37, 44, 45, 46, 47, 48, 49, 50, 51, 52]. Nevertheless, despite several similar results, some studies did not find any changes in NAA after TBI [53, 54]. We believe that the main reason for these results was the prolonged interval between injury and imaging [55, 56]. These data do not role out hyperacute metabolite alterations and the value of early metabolite alterations in clinical evaluations [53].
Choline (Cho) or total choline includes free choline, glycerol-phosphocholine, and phosphocholine. It is observed as a prominent singlet at 3.2 ppm in the H-MRS [11, 13]. However, the range of Cho changes is too tiny. Such changes may associate with changes in membrane turnover or brain injuries [13]. Regarding the literature, the increase in Cho level in the TBI group compared with the healthy group is due to tissue breakdown [9, 62, 63]; Recently, Babikian et al. argued that lower lobar Cho levels in the chronic stage of TBI were correlated with declined Interhemispheric Transfer Time (IHTT) in moderate to severe TBI subgroups [69]. Nevertheless, probably Cho levels would be higher in more severe TBI cases, compared to a healthy population; however, the lower level of Cho in patients with more severe neurocognitive outcomes, compared to patients with better outcomes may be attributed to decreased membrane turnover in the setting of permanent cellular damage [28, 55].
Creatine (Cr) is an indirect intermediator of the energy used as a marker for the total stored energy of the cell [57, 58]. Cr peak resonates at 3.03 ppm [13]. A decrease in Cr in the traumatic infarction area supports dropping Cr level when the cell is dead [59]. Yeo et al. found a significant negative correlation between Cr and days after injury, suggesting that damaged cell repair occurs using cell storage energy [53]. Of note, an interval of 3 to 5 months for complete biochemically repairing of the injured cells suggests that this interval may associate with Cr level base as a marker for cellular energy [29].
The MI is another metabolite that can be detected using short echo time spectroscopy [31]. MI, known as an osmolyte, increases parallel to activation of microgliosis following TBI. Contrary to NAA, Cr, and Cho, which have a trend toward average over time, it is believed that MI level increases [6]. Still, there is no evidence of significant MI changes on the day of brain injury. Since MI is a gliotic marker, an increase in MI is expected in subacute and chronic TBI stages [6]. It has to be mentioned that some extra-metabolites are less common to calculate by MRS due to their very low concentration in the brain. Glutamate, glutamine, glutathione, GABA, and lactate are important extra metabolites that MRS can detect.
Glutamate (Glu) is an excitatory neurotransmitter that converts to glutamine (Gln) within astrocytes. Because most clinical MRI scanners are 1.5 T, Glu and Gln are usually reported in combination due to their overlapping resonances [60]. Elevated Glx and Glu following TBI are supposed to happen due to excitotoxicity [29]
Glutathione is a tripeptide thiol that acts as an oxidation-reduction cofactor in enzyme reactions [61].
γ-Aminobutyric acid (GABA) is the main inhibitory neurotransmitter in the human cortex. Literature indicates that GABA may play a potential role in cognitive dysfunctions following TBI [62]. A few previous translational studies have suggested that neurotransmitters, including acetylcholine, glutamate, dopamine, serotonin, and GABA, may potentially be biomarkers of cognitive dysfunctions in TBI [40]. Lactate occupies a unique position in energy metabolism. It is believed that the presentation of lactate in MRS suggests a poorer prognosis [63].
Absolute Neuro-Metabolites and Post-Traumatic Neurocognitive Disorders
N-Acetylaspartate (NAA) is the commonest neuro-metabolite assessed by the MRS. The majority of studies found a lower level of NAA in traumatic patients compared with control groups in various parts of the brain [32]. Research supports a strong relationship between decreased NAA level and neurocognitive outcomes [22, 36, 40, 64, 65, 66]. According to a study performed by Babikian et al., NAA explains over 40% of the variance in long-term outcomes of the cognitive functions [36]. MacMaster et al. found a significant association between decreased NAA in the left dorsolateral prefrontal cortex (DLPFC) and emotional symptoms in youth with a concussion in a recent study. In addition, a significant negative correlation exists between NAA level and emotional stress degree [26]. Previously, Sivak et al. reported a significant correlation between NAA in left DLPFC and computerized cognitive tests (backward digit span, Stroop test A, TMT-B time) [27].
However, most studies did not report a significant correlation between neuro-metabolites and computerized tests. Some authors reported a significant negative correlation between NAA and computerized cognitive tests. Babikian et al. noted a positive correlation between NAA in the posterior part of the corpus callosum and several neurocognitive measures, reaching statistical significance for FSIQ, visual memory, and processing speed [38]. Although longitudinal studies support that increased NAA level towards a normal value after 30 days of injury is related to better long-term outcomes [67].
Choline (Cho) increase after TBI has been reported by some studies [37]. Some are reported the association between the increase mentioned above with insignificant outcomes [23, 64, 68]. In contrast, Parry et al. found that lower levels of Cho displayed reduced performances on neurocognitive tests [23]. Babikian et al. evaluated the correlation between single-voxel spectroscopy (SVS) data from the anterior and posterior part of the corpus callosum and neurocognitive performance. In this regard, the authors reported a positive correlation in the anterior part for response time, while in the posterior part, they concluded that this metabolite was statistically significant for FSIQ and approached significance for visual memory [38].
In a more recent study, Babikian et al. showed that lower lobar Cho levels in the chronic stage of TBI were correlated with lower interhemispheric transfer time (IHTT) in moderate to severe TBI subgroups [69].
Nevertheless, We believe that probably Cho level would be higher in more severe TBI than in a healthy population; but the lower level of Cho in the patients with more severe neurocognitive outcomes comparing patients with better outcomes may be due to decreased membrane turnover in the setting of permanent cellular damage [28, 55].
Few specialists believe that the creatine (Cr) level is stable in different situations [38], while some studies report that Cr can be changed significantly due to different situations [2, 29]. Gasparovic et al. introduced a significant regression model to predict emotional disorders based on Cr level in the splenium of the CC and white matter of the cingulate gyrus [29]. In addition, George et al. and Babikian et al. found a significant positive correlation between the acute phase of Cr level and neurocognitive outcomes in traumatic brain injuries [2, 38].
Current studies showed more interest in detecting MI in the sub-acute and chronic phases after traumatic brain injuries [20, 21, 31]. In this context, Ashwal et al. found a significant association between higher MI levels in occipital gray matter and poorer outcomes 6-12 months after injury [31]. Recently Alosco et al. published a positive correlation between MI from anterior cingulate cortex and behavioral/mood symptoms in patients with repetitive impact injury history comparing non-exposed control groups [20]. A study performed by Gardner et al. showed that in retired athletes, significant positive correlations between DASS (depression anxiety stress scale) and grey matter MI suggest that increasing MI levels are associated with worse anxiety symptoms in the posterior cingulate cortex [21]. In addition, the authors also reported a significant correlation between RPQ score and MI level in this area [21].
There are some neuro-metabolites which MRS does not usually detect. A few studies reported significant associations between GLx from occipital gray matter and parietal white matter [23]. Shutter et al. indicate that Cho and GLx have 94% accuracy in predicting long-term traumatic brain injuries [23]. Recently, Sheth et al. reported a lower glutamine level in the dorsal anterior cingulate cortex [19]. Besides, few studies have demonstrated a positive correlation between glutamate, glutathione, and behavioral/mood symptoms [20, 69]. Gardner et al. designed a study to examine if brain neuro-metabolite concentrations are associated with neurocognitive disorders in retired rugby league players who had a history of numerous self-reported concussions. The authors performed MRS in the posterior cingulate cortex and parietal white matter using 3 T single-voxel spectroscopy. The results showed that DASS anxiety scores were positively correlated with grey matter glutamate and Glx. Also, Rivermead post-concussion syndrome scores were positively correlated with grey matter glutamate. In addition, BESS scores were negatively correlated with grey matter glutathione and white matter glutathione [21].
Another interested extra-metabolite is GABA. Some studies support the role of GABA in post-traumatic stress disorders [19]. In a recent study, Kim et al. showed that the GABA level in the prefrontal cortex was correlated with memory performance in the Boxers but not in attention performance [24]. Lastly, some studies report that the presence of high lactate in patients with traumatic brain injuries had weaker association outcomes [30, 37].
Relative Neuro-Metabolites and Post-Traumatic Neurocognitive Disorders
Some experts believe that the relative value of metabolites is more reliable than the absolute value of metabolites [58]. Although there are some controversies, some authors believe that the Cr level remains stable in pathologic situations. Thus, most studies have used Cr as a base for relative measures [2, 27, 35, 41].
Sivak et al. found significant correlations between NAA/Cr in the right DLPFC and neurocognitive tests (total digit span and Stroop test) [27]. George et al. showed that NAA/Cr ratio in the thalamus is significantly associated with cognitive tests [2]. Likewise, many studies reported an association between reductions in NAA/Cr and poor long-term neurocognitive performance [30, 41]. NAA/Cho is another ratio that has been reported as a valuable relationship with poor long-term neurological outcomes [30, 32].
Cho/Cr is another essential ratio in the setting of TBI. An increasing trend of Cho/Cr in DLPFC in patients with PCS is reported by Dean et al. [25]. Another study by Dean et al. showed an increase in Cho/Cr ratio is correlated with the Rivermead Post-Concussion Syndrome Questionnaire Score (RPQS) [71]. Nevertheless, some studies report that Cho/Cr ratio is higher in traumatic groups with poorer outcomes [30].
A chemical shift imaging study performed by Yeo et al. on Cho/Cr ratios from the frontal lobe to the posterior parietal lobes and sampling from both white and gray matter [41]. Thus, the relationship was confirmed in other parts of the brain, such as the thalamus [35].
Conclusion
We found that MRS data can provide essential data for clinicians to predict neurocognitive outcomes following traumatic brain injuries. Neuro-metabolite ratios provide a broader information to evaluate cellular integrity in brain tissue. Further studies are needed to prepare predictive models based on MRS data for post-traumatic neurocognitive disorders.
Ethical Considerations
Compliance with ethical guidelines
All study procedures were done in compliance with the ethical guidelines of the 2013 version of the Declaration of Helsinki.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
Conceptualization and Supervision: Mohammad Haghani Dogahe; Methodology: Mohammad Haghani Dogahe and Alireza Feizkhah; Investigation: Mohammad Haghani Dogahe, Alireza Feizkhah, and Sara Seddighi; Writing the original draft, review, and editing: All authors.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank Dr. Mohammadreza Mobayen (Burn and Regenerative Medicine Research Center, Guilan University of Medical Sciences, Rasht City, Iran) for his expert advice and encouragement throughout this project and for providing a suitable place for researchers.
References
- Belanger HG, Vanderploeg RD, Curtiss G, Warden DL. Recent neuroimaging techniques in mild Traumatic Brain Injury. J Neuropsychiatry Clin Neurosci. 2007; 19(1):5-20. [DOI:10.1176/jnp.2007.19.1.5] [PMID]
- George EO, Roys S, Sours C, Rosenberg J, Zhuo J, Shanmuganathan K, et al. Longitudinal and prognostic evaluation of mild Traumatic Brain Injury: A 1H-magnetic resonance spectroscopy study. J neurotrauma. 2014; 31(11):1018-28. [DOI:10.1089/neu.2013.3224] [PMID]
- Sullivan PG, Geiger JD, Mattson MP, Scheff SW. Dietary supplement creatine protects against Traumatic Brain Injury. Ann Neurol. 2000; 48(5):723-9. [DOI:10.1002/1531-8249(200011)48:53.0.CO; 2-W]
- Narayana PA, Yu X, Hasan KM, Wilde EA, Levin HS, Hunter JV, et al. Multi-modal MRI of mild Traumatic Brain Injury. NeuroImage: Clinical. 2015; 7:87-97. [DOI:10.1016/j.nicl.2014.07.010] [PMID] [PMCID]
- Egerton A. The potential of 1H-MRS in CNS drug development. Psychopharmacology (Berl). 2019; 238(5):1241-54. [DOI:10.1007/s00213-019-05344-7] [PMID] [PMCID]
- Kierans AS, Kirov, II, Gonen O, Haemer G, Nisenbaum E, Babb JS, et al. Myoinositol and glutamate complex neurometabolite abnormality after mild Traumatic Brain Injury. Neurology. 2014; 82(6):521-8. [DOI:10.1212/WNL.0000000000000105] [PMID] [PMCID]
- Gennarelli TA. Mechanisms of brain injury. J Emerg Med. 1993; 11(suppl 1):5-11. [PMID]
- Jawad M, Evers M, Gerwing A, Herick M, Seibert D, Bauer J, et al. A visual analytics approach for comparing cohorts in single-voxel magnetic resonance spectroscopy data. Adv Exp Med Biol. 2019; 1138:115-36. [DOI:10.1007/978-3-030-14227-8_9] [PMID]
- Geurts JJ, Barkhof F, Castelijns JA, Uitdehaag BM, Polman CH, Pouwels PJ. Quantitative 1H-MRS of healthy human cortex, hippocampus, and thalamus: Metabolite concentrations, quantification precision, and reproducibility. J Magn Reson Imaging. 2004; 20(3):366-71. [DOI:10.1002/jmri.20138] [PMID]
- Faghihi R, Zeinali-Rafsanjani B, Mosleh-Shirazi MA, Saeedi-Moghadam M, Lotfi M, Jalli R, et al. Magnetic resonance spectroscopy and its clinical applications: A review. J Med Imaging Radiat Sci. 2017; 48(3):233-53. [DOI:10.1016/j.jmir.2017.06.004] [PMID]
- Mazzoni LN, Belli G, Ginestroni A, Pratesi A, Agnoloni S, Diciotti S, et al. Computation of brain metabolite ratios in single-voxel proton MR spectroscopy: Comparison between semiautomatic and automatic software. Radiol Med. 2010; 115(1):125-32. [DOI:10.1007/s11547-009-0408-4] [PMID]
- Waagepetersen HS, Sonnewald U, Schousboe A. 1 Glutamine, Glutamate, and GABA: Metabolic aspects. In: Lajtha A, Oja SS, Schousboe A, Saransaari P, editors. Handbook of Neurochemistry and Molecular Neurobiology: Amino Acids and Peptides in the Nervous System. Boston, MA: Springer US; 2007. pp. 1-21. [DOI:10.1007/978-0-387-30373-4_1]
- Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000; 13(3):129-53. [DOI:10.1002/1099-1492(200005)13:33.0.CO; 2-V]
- Coxon JP, Cash RFH, Hendrikse JJ, Rogasch NC, Stavrinos E, Suo C, et al. GABA concentration in sensorimotor cortex following high-intensity exercise and relationship to lactate levels. J Physiol. 2018; 596(4):691-702. [DOI:10.1113/JP274660] [PMID] [PMCID]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical research ed). 2009; 339:b2700. [DOI: 10.1136/bmj.b2700] [PMID] [PMCID]
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016; 355. [DOI:10.1136/bmj.i4919] [PMID] [PMCID]
- Andrews JC, Schünemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, et al. GRADE guidelines: 15. Going from evidence to recommendation - Determinants of a recommendation’s direction and strength. J Clin Epidemiol. 2013; 66(7):726-35. [DOI:10.1016/j.jclinepi.2012.03.013]
- Zhang Y, Taub E, Salibi N, Uswatte G, Maudsley AA, Sheriff S, et al. Comparison of reproducibility of single voxel spectroscopy and whole-brain magnetic resonance spectroscopy imaging at 3T. NMR Biomed. 2018; 31(4):e3898. [DOI:10.1002/nbm.3898] [PMID] [PMCID]
- Sheth C, Prescot AP, Legarreta M, Renshaw PF, McGlade E, Yurgelun-Todd D. Reduced gamma-amino butyric acid (GABA) and glutamine in the Anterior Cingulate Cortex (ACC) of veterans exposed to trauma. J Affect Disord. 2019; 248:166-74. [DOI:10.1016/j.jad.2019.01.037] [PMID]
- Alosco ML, Tripodis Y, Rowland B, Chua AS, Liao H, Martin B, et al. A magnetic resonance spectroscopy investigation in symptomatic former NFL players. Brain Imaging Beh. 2020; 14(5):1419-29. [DOI:10.1007/s11682-019-00060-4] [PMID]
- Gardner AJ, Iverson GL, Wojtowicz M, Levi CR, Kay-Lambkin F, Schofield PW, et al. MR spectroscopy findings in retired professional rugby league players. Int J Sports Med. 2017; 38(03):241-52. [DOI:10.1055/s-0042-120843] [PMID]
- Parry L, Shores A, Rae C, Kemp A, Waugh M-C, Chaseling R, et al. An investigation of neuronal integrity in severe paediatric Traumatic Brain Injury. Child Neuropsychol. 2004; 10(4):248-61. [DOI:10.1080/09297040490909279] [PMID]
- Shutter L, Tong KA, Holshouser BA. Proton MRS in acute Traumatic Brain Injury: Role for glutamate/glutamine and choline for outcome prediction. J Neurotrauma. 2004; 21(12):1693-705. [DOI:10.1089/neu.2004.21.1693] [PMID]
- Kim GH, Kang I, Jeong H, Park S, Hong H, Kim J, et al. Low prefrontal GABA levels are associated with poor cognitive functions in professional boxers. Front Hum Neurosci. 2019; 13:193. [DOI:10.3389/fnhum.2019.00193] [PMID] [PMCID]
- Dean PJ, Sato JR, Vieira G, McNamara A, Sterr A. Multimodal imaging of mild Traumatic Brain Injury and persistent post-concussion syndrome. Brain and behavior. 2015; 5(1):e00292. [DOI:10.1002/brb3.292] [PMID] [PMCID]
- Macmaster FP, McLellan Q, Harris AD, Virani S, Barlow KM, Langevin LM, et al. N-acetyl-aspartate in the dorsolateral prefrontal cortex long after concussion in youth. J Head Trauma Rehabil. 2020; 35(2):E127-E35. [DOI:10.1097/HTR.0000000000000535] [PMID]
- Sivák S, Bittšanský M, Grossmann J, Nosál V, Kantorová E, Siváková J, et al. Clinical correlations of proton magnetic resonance spectroscopy findings in acute phase after mild Traumatic Brain Injury. Brain Inj. 2014; 28(3):341-6. [DOI:10.3109/02699052.2013.865270] [PMID]
- Panchal H, Sollmann N, Pasternak O, Alosco ML, Kinzel P, Kaufmann D, et al. Neuro-metabolite changes in a single season of university ice hockey using magnetic resonance spectroscopy. Front Neurol. 2018; 9:616. [DOI:10.3389/fneur.2018.00616] [PMID] [PMCID]
- Gasparovic C, Yeo R, Mannell M, Ling J, Elgie R, Phillips J, et al. Neurometabolite concentrations in gray and white matter in mild Traumatic Brain Injury: An 1H-magnetic resonance spectroscopy study. J neurotrauma. 2009; 26(10):1635-43. [DOI:10.1089/neu.2009.0896] [PMID] [PMCID]
- Brenner T, Freier MC, Holshouser BA, Burley T, Ashwal S. Predicting neuropsychologic outcome after Traumatic Brain Injury in children. PediatrNeurol. 2003; 28(2):104-14. [DOI:10.1016/S0887-8994(02)00491-5]
- Ashwal S, Holshouser B, Tong K, Serna T, Osterdock R, Gross M, et al. Proton spectroscopy detected myoinositol in children with Traumatic Brain Injury. Pediatric Research. 2004; 56(4):630-8. [DOI:10.1203/01.PDR.0000139928.60530.7D] [PMID]
- Ariza M, Junqué C, Mataró M, Poca MA, Bargalló N, Olondo M, et al. Neuropsychological correlates of basal ganglia and medial temporal lobe NAA/Cho reductions in Traumatic Brain Injury. Arch Neurol. 2004; 61(4):541-4. [DOI:10.1001/archneur.61.4.541] [PMID]
- Shah A, Jhawar SS, Goel A. Analysis of the anatomy of the Papez circuit and adjoining limbic system by fiber dissection techniques. J Clinl Neurosci. 2012; 19(2):289-98. [DOI:10.1016/j.jocn.2011.04.039] [PMID]
- Holshouser BA, Tong KA, Ashwal S, Oyoyo U, Ghamsary M, Saunders D, et al. Prospective longitudinal proton magnetic resonance spectroscopic imaging in adult Traumatic Brain Injury. J Magn Reson Imaging. 2006; 24(1):33-40. [DOI:10.1002/jmri.20607] [PMID]
- Sours C, George EO, Zhuo J, Roys S, Gullapalli RP. Hyper-connectivity of the thalamus during early stages following mild Traumatic Brain Injury. Brain Imaging Behav. 2015; 9(3):550-63. [DOI:10.1007/s11682-015-9424-2] [PMID] [PMCID]
- Babikian T, Freier MC, Ashwal S, Riggs ML, Burley T, Holshouser BA. MR spectroscopy: Predicting long‐term neuropsychological outcome following pediatric TBI. J Magn Reson Imaging. 2006; 24(4):801-11. [DOI:10.1002/jmri.20696] [PMID]
- Marino S, Zei E, Battaglini M, Vittori C, Buscalferri A, Bramanti P, et al. Acute metabolic brain changes following Traumatic Brain Injury and their relevance to clinical severity and outcome. J Neurol Neurosurg Psychiatry. 2007; 78(5):501-7. [DOI:10.1136/jnnp.2006.099796] [PMID] [PMCID]
- Babikian T, Marion SD, Copeland S, Alger JR, O’Neill J, Cazalis F, et al. Metabolic levels in the corpus callosum and their structural and behavioral correlates after moderate to severe pediatric TBI. J Neurotrauma. 2010; 27(3):473-81. [DOI:10.1089/neu.2009.1058] [PMID] [PMCID]
- Tang S, Xu S, Fourney WL, Leiste UH, Proctor JL, Fiskum G, et al. Central nervous system changes induced by underbody blast-induced hyperacceleration: An in vivo diffusion tensor imaging and magnetic resonance spectroscopy study. J Neurotrauma. 2017; 34(11):1972-80. [DOI:10.1089/neu.2016.4650] [PMID]
- Kirov II, Tal A, Babb JS, Lui YW, Grossman RI, Gonen O. Diffuse axonal injury in mild Traumatic Brain Injury: A 3D multivoxel proton MR spectroscopy study. J Neurol. 2013; 260(1):242-52. [DOI:10.1007/s00415-012-6626-z] [PMID] [PMCID]
- Yeo RA, Phillips JP, Jung RE, Brown AJ, Campbell RC, Brooks WM. Magnetic resonance spectroscopy detects brain injury and predicts cognitive functioning in children with brain injuries. J neurotrauma. 2006; 23(10):1427-35. [DOI:10.1089/neu.2006.23.1427] [PMID]
- Deelchand DK, Adanyeguh IM, Emir UE, Nguyen TM, Valabregue R, Henry PG, et al. Two-site reproducibility of cerebellar and brainstem neurochemical profiles with short-echo, single-voxel MRS at 3T. Magn Reson Med. 2015; 73(5):1718-25. [DOI:10.1002/mrm.25295] [PMID] [PMCID]
- Li W, Xiang Q, Liu D, Li Y. Disrupted coupling between naa and functional connectivity in ventromedial prefrontal cortex of drug-naïve first-episode psychosis. Annu Int Conf IEEE Eng Med Biol Soc. 2020; 2020:1738-41. [DOI:10.1109/EMBC44109.2020.9176293] [PMID]
- Veeramuthu V, Seow P, Narayanan V, Wong JHD, Tan LK, Hernowo AT, et al. Neurometabolites alteration in the acute phase of mild Traumatic Brain Injury (mTBI): An in vivo proton Magnetic Resonance Spectroscopy (1H-MRS) Study. Acad Radiol. 2018; 25(9):1167-77. [DOI:10.1016/j.acra.2018.01.005] [PMID]
- Vagnozzi R, Signoretti S, Tavazzi B, Floris R, Ludovici A, Marziali S, et al. Temporal window of metabolic brain vulnerability to concussion: a pilot 1H-magnetic resonance spectroscopic study in concussed athletes--part III. Neurosurg Sci time. 2008; 62(6):1286-95; discussion 95-6. [DOI:10.1227/01.neu.0000333300.34189.74] [PMID]
- Vagnozzi R, Signoretti S, Cristofori L, Alessandrini F, Floris R, Isgrò E, et al. Assessment of metabolic brain damage and recovery following mild Traumatic Brain Injury: A multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain. 2010; 133(11):3232-42. [DOI:10.1093/brain/awq200] [PMID]
- Kirov, II, Tal A, Babb JS, Reaume J, Bushnik T, Ashman TA, et al. Proton MR spectroscopy correlates diffuse axonal abnormalities with post-concussive symptoms in mild Traumatic Brain Injury. J Neurotrauma. 2013; 30(13):1200-4. [DOI:10.1089/neu.2012.2696] [PMID] [PMCID]
- Son BC, Park CK, Choi BG, Kim EN, Choe BY, Lee KS, et al. Metabolic changes in pericontusional oedematous areas in mild head injury evaluated by 1H MRS. Acta Neurochir Suppl. 2000; 76:13-6. [DOI:10.1007/978-3-7091-6346-7_3] [PMID]
- Macmillan CS, Wild JM, Wardlaw JM, Andrews PJ, Marshall I, Easton VJ. Traumatic brain injury and subarachnoid hemorrhage: In vivo occult pathology demonstrated by magnetic resonance spectroscopy may not be “ischaemic”. A primary study and review of the literature. Acta Neurochir (Wien). 2002; 144(9):853-62; discussion 62. [DOI:10.1007/s00701-002-0966-x] [PMID]
- Govindaraju V, Gauger GE, Manley GT, Ebel A, Meeker M, Maudsley AA. Volumetric proton spectroscopic imaging of mild Traumatic Brain Injury. Am J Neuroradiol (AJNR). 2004; 25(5):730-7. [PMID] [PMCID]
- Uzan M, Albayram S, Dashti S, Aydin S, Hanci M, Kuday C. Thalamic proton magnetic resonance spectroscopy in vegetative state induced by Traumatic Brain Injury. J Neurol Neurosurg Psychiatry. 2003; 74(1):33-8. [DOI:10.1136/jnnp.74.1.33] [PMID] [PMCID]
- Govind V, Gold S, Kaliannan K, Saigal G, Falcone S, Arheart KL, et al. Whole-brain proton MR spectroscopic imaging of mild-to-moderate Traumatic Brain Injury and correlation with neuropsychological deficits. J Neurotrauma. 2010; 27(3):483-96. [DOI:10.1089/neu.2009.1159] [PMID] [PMCID]
- Yeo RA, Gasparovic C, Merideth F, Ruhl D, Doezema D, Mayer AR. A longitudinal proton magnetic resonance spectroscopy study of mild Traumatic Brain Injury. J Neurotrauma. 2011; 28(1):1-11. [DOI:10.1089/neu.2010.1578] [PMID] [PMCID]
- Maugans TA, Farley C, Altaye M, Leach J, Cecil KM. Pediatric sports-related concussion produces cerebral blood flow alterations. Pediatrics. 2012; 129(1):28-37. [DOI:10.1542/peds.2011-2083] [PMID] [PMCID]
- De Stefano N, Matthews PM, Arnold DL. Reversible decreases in N-acetylaspartate after Acute Brain Injury. Magn Reson Med. 1995; 34(5):721-7. [DOI:10.1002/mrm.1910340511] [PMID]
- Schuhmann MU, Stiller D, Skardelly M, Bernarding J, Klinge PM, Samii A, et al. Metabolic changes in the vicinity of brain contusions: A proton magnetic resonance spectroscopy and histology study. J Neurotrauma. 2003; 20(8):725-43. [DOI:10.1089/089771503767869962] [PMID]
- Li Y, Lafontaine M, Chang S, Nelson SJ. Comparison between short and long echo time magnetic resonance spectroscopic imaging at 3T and 7T for evaluating brain metabolites in patients with glioma. ACS Chem Neurosci. 2018; 9(1):130-7. [DOI:10.1021/acschemneuro.7b00286] [PMID] [PMCID]
- Pinggera D, Steiger R, Bauer M, Kerschbaumer J, Luger M, Beer R, et al. Cerebral energy status and altered metabolism in early severe TBI: First results of a prospective 31 P-MRS feasibility study. Neurocrit Care. 2020:1-9. [DOI:10.1007/s12028-020-01042-x] [PMID]
- Sutton L, Wang Z, Duhaime A, Costarino D, Sauter R, Zimmerman R. Tissue lactate in pediatric head trauma: A clinical study using 1H NMR spectroscopy. Pediatrics. 1995; 22(2):81-7. [DOI:10.1159/000120881] [PMID]
- Hancu I. Optimized glutamate detection at 3T. J Magn Reson Imaging (JMRI). 2009; 30(5):1155-62. [DOI:10.1002/jmri.21936] [PMID] [PMCID]
- Rae CD, Williams SR. Glutathione in the human brain: Review of its roles and measurement by magnetic resonance spectroscopy. Anal Biochem. 2017; 529:127-43. [DOI:10.1016/j.ab.2016.12.022] [PMID]
- Boonstra E, de Kleijn R, Colzato LS, Alkemade A, Forstmann BU, Nieuwenhuis S. Neurotransmitters as food supplements: The effects of GABA on brain and behavior. Front Psychol. 2015; 6:1520. [DOI:10.3389/fpsyg.2015.01520] [PMID] [PMCID]
- Prichard JW. What the clinician can learn from MRS lactate measurements. NMR Biomed. 1991; 4(2):99-102. [DOI:10.1002/nbm.1940040212] [PMID]
- Brooks WM, Stidley CA, Petropoulos H, Jung RE, Weers DC, Friedman SD, et al. Metabolic and cognitive response to human Traumatic Brain Injury: A quantitative proton magnetic resonance study. J Neurotrauma. 2000; 17(8):629-40. [DOI:10.1089/089771500415382] [PMID]
- Carpentier A, Galanaud D, Puybasset L, Muller J-C, Lescot T, Boch A-L, et al. Early morphologic and spectroscopic magnetic resonance in severe traumatic brain injuries can detect “invisible brain stem damage” and predict “vegetative states”. J Neurotrauma. 2006; 23(5):674-85. [DOI:10.1089/neu.2006.23.674] [PMID]
- Friedman S, Brooks W, Jung R, Chiulli S, Sloan J, Montoya B, et al. Quantitative proton MRS predicts outcome after Traumatic Brain Injury. Neurology. 1999; 52(7):1384-91. [DOI:10.1212/WNL.52.7.1384] [PMID]
- Signoretti S, Marmarou A, Fatouros P, Hoyle R, Beaumont A, Sawauchi S, et al. Application of chemical shift imaging for measurement of NAA in head injured patients. Acta Neurochir Suppl. 2002; 81:373-5. [DOI:10.1007/978-3-7091-6738-0_94] [PMID]
- Shutter L, Tong KA, Lee A, Holshouser BA. Prognostic role of proton magnetic resonance spectroscopy in acute Traumatic Brain Injury. J Head Trauma Rehabil. 2006; 21(4):334-49. [DOI:10.1097/00001199-200607000-00005] [PMID]
- Babikian T, Alger JR, Ellis-Blied MU, Giza CC, Dennis E, Olsen A, et al. Whole brain magnetic resonance spectroscopic determinants of functional outcomes in pediatric moderate/ severe traumatic brain injury. J Neurotrauma. 2018; 35(14):1637-45. [DOI: 10.1089/neu.2017.5366] [PMID]
- De Beaumont L, Tremblay S, Henry LC, Poirier J, Lassonde M, Théoret H. Motor system alterations in retired former athletes: the role of aging and concussion history. BMC neurology. 2013; 13(1):109. https://bmcneurol.biomedcentral.com/articles/10.1186/1471-2377-13-109
- Dean PJ, Otaduy MC, Harris LM, McNamara A, Seiss E, Sterr A. Monitoring long-term effects of mild Traumatic Brain Injury with magnetic resonance spectroscopy: A pilot study. Neuroreport. 2013; 24(12):677-81. [DOI:10.1097/WNR.0b013e3283637aa4] [PMID]
Type of Study: Review |
Subject:
Special
Received: 2021/07/1 | Accepted: 2021/04/30 | Published: 2021/04/30
Received: 2021/07/1 | Accepted: 2021/04/30 | Published: 2021/04/30
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






.jpg)
