Wed, Apr 24, 2024
Volume 7, Issue 1 (Winter 2021)
Caspian J Neurol Sci 2021, 7(1): 1-9 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Keymoradzadeh A, Komaki A, Bakhshi A, Faraji N, Golipoor Z, Shahshahani P. The Effect of Different Doses of Melatonin on Learning and Memory Deficit in Alzheimer Model of Rats. Caspian J Neurol Sci 2021; 7 (1) :1-9
URL: http://cjns.gums.ac.ir/article-1-382-en.html
URL: http://cjns.gums.ac.ir/article-1-382-en.html
The Effect of Different Doses of Melatonin on Learning and Memory Deficit in Alzheimer Model of Rats
Arman Keymoradzadeh1 

 , Alireza Komaki2
, Alireza Komaki2 

 , Arash Bakhshi1
, Arash Bakhshi1 

 , Nafise Faraji2
, Nafise Faraji2 

 , Zoleikha Golipoor *
, Zoleikha Golipoor * 

 3, Parisa Shahshahani4
3, Parisa Shahshahani4 



 , Alireza Komaki2
, Alireza Komaki2 

 , Arash Bakhshi1
, Arash Bakhshi1 

 , Nafise Faraji2
, Nafise Faraji2 

 , Zoleikha Golipoor *
, Zoleikha Golipoor * 

 3, Parisa Shahshahani4
3, Parisa Shahshahani4 

1- Student Research Committee, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
2- Neurophysiology Research Center, Hamadan University of Medical Sciences, Hamadan, Iran.
3- Neuroscience Research Center, Guilan University of Medical Sciences, Rasht, Iran. , masoomeh_golipoor@yahoo.com
4- Department of Neurology, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
2- Neurophysiology Research Center, Hamadan University of Medical Sciences, Hamadan, Iran.
3- Neuroscience Research Center, Guilan University of Medical Sciences, Rasht, Iran. , masoomeh_golipoor@yahoo.com
4- Department of Neurology, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
Full-Text [PDF 2262 kb]
(747 Downloads)
| Abstract (HTML) (1617 Views)
Full-Text: (526 Views)
Introduction
lzheimer disease (AD) is an age-related neurodegenerative disorder characterized by a progressive impairment of cognitive function [1, 2]. AD targets vital brain cells through which it affects thinking, memory, and behavior: these effects compromise work and social life [3].
Pathogenesis of AD has not been fully understood; however, it is evident that amyloid-βeta (Aβ) accumulation is an integral part of this disease [4], as it plays an essential role in the AD-related neurodegenerative process [3]. Aβ is a peptide containing 37–43 amino acids, and it is produced from Amyloid Precursor Protein (APP) [5] through a proteolytic cleavage mediated by β- and γ-secretases [4]. Intraventricular injection of Aβ is known to induce learning and memory deficits [3]. Toxic effects of Aβ in the brain may exert through an increase in neuroinflammation, mitochondrial dysfunction, and Reactive Oxygen Species (ROS), through which it induces oxidative stress in the brain [4, 6, 7, 8]. Oxidative stress plays a key role in learning and memory impairment [9].
Melatonin (MEL) (N-acetyl-5-methoxytryptamine) is a production of the pineal gland [10] and a multifunctional molecule that participate in a variety of physiological functions, including antioxidant [11, 12, 13, 14] and radical scavenger against harmful effects of free radicals on biological membrane lipids, Deoxyribonucleic Acid (DNA), and proteins [12 ,15]. Melatonin appears effective and safe in improving sleep quality in patients with AD [16]. There is growing evidence that sheds light on the protective role of MEL in memory deficit [9], aging, and Alzheimer disease [17]. MEL can be served as a neuroprotective hormone against mitochondrial damage [18], oxidative stress resulting in amelioration of learning deficits and neurodegenerative diseases [19, 20]. MEL has anti-amyloid properties through inhibition of Aβ generation [21]. Also, MEL stimulates the nonamyloidogenic pathway over the amyloidogenic pathway in the cultured neuronal and non-neuronal cells [22, 23, 24].
It has been suggested that melatonin has sufficient high concentrations in the cerebral tissue [25], and melatonin analogs are employed in higher densities [26]. However, different doses of melatonin have been shown to have different effects on memory in animal models of memory impairment; for example, one study indicated that melatonin at a dose of 10 mg/kg improves memory [27], while another study found no effect on memory [28]. In another study, a dose of 20 mg/kg melatonin improved memory [29], suggesting that doses greater than 10 mg/kg may improve memory, whereas, in their study, a dose of 30 mg did not affect memory [30]. Thus, this study aimed to evaluate the treatment role of two different MEL dosages on the learning and memory, Amyloid Precursor Protein (APP) deposit and migration of microglia in AD rats.
Materials and Methods
Animals
A total of 48 male Wistar rats weighing 250-300 g were obtained from the Medical Faculty of Hamedan University of Medical Sciences. The rats were placed in standard conditions of illumination (12 h light-dark cycle), humidity (55%-65%), and temperature (22°C). They were allowed to have easy access to food and water ad libitum for one week to be acclimatized to the new environment. All procedures were performed by reliance on the guides from the Ethics Committee of Hamedan University of Medical Sciences compliance to the institutional and national guidelines for animal care and use.
Experimental design
Animals were divided randomly into 6 groups, including control, sham, vehicle, AD, AD+MEL10, and AD+MEL 20. AD received Aβ1-42 on the first day of experiments. After two weeks, MEL (Sigma) at two doses of 10 mg/kg and 20 mg/kg was administered intraperitoneally once per week for 4 weeks [28, 29]. Then, the learning and spatial memory of the rats were evaluated. Morris Water Maze (MWM) and Passive Avoidance Learning (PAL) tests were used to assess memory function. Also, APP microglia was assessed by histological study, respectively.
Surgery and injection of Aβ (1–42)
The procedure was done according to previous studies [31, 32]. The animals were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and submitted to a stereotaxic apparatus (Stoelting, USA). According to the bregma, the coordinates for the bilateral intracerebroventricular were AP: 1.2 mm; ML: 2 mm; and DV: 4 mm [33]. Aβ (Tocris Bioscience, UK) solution (1 µg/µL, 5 μL) was bilaterally microinjected into the region. Sham rats only sustained surgery procedure without any injection. Vehicle groups received the same volume of vehicle. Following injections, the skin was sutured, and the animals were allowed to recover in their home cages.
Passive Avoidance Learning (PAL)
Apparatus
PAL was evaluated using a step-through apparatus [34] consisted of two compartments: a light compartment (20 cm × 20 cm × 30 cm) made from transparent plastic and a dark compartment made from dark opaque plastic (20 cm × 20 cm × 30 cm). A stainless steel rod with a diameter of 3 mm was applied to the floor of the compartments. A shock generator (Behbood Pardaz Co. Tehran, Iran) was used to electrify the dark compartment floor. A rectangular opening (6 cm × 8 cm) capable of closing by an opaque guillotine door was located between the two compartments [34].
Training
First, each rat received two trials to become familiarized with the apparatus. For this purpose, the rats were located in the light compartment facing away from the door for 5 s. Then, the guillotine door was raised. Typically, the rats have an instinctive tendency to the dark environment. When the rats entered the dark compartment, the guillotine door was closed, and the animals were kept for 30 s in this compartment. This trial was repeated after 30 min interval. The entrance latency to the dark compartment (STLa: Step-through latency in the acquisition) was tested when the rats were placed in the dark compartment [35].
After the rats entered the dark compartment spontaneously, the guillotine door was closed for 30 s, and a 0.5 mA electrical shock was applied for 3 s. The procedure was repeated after 2 min. The training was finished when animals stayed in the light compartment for 120 consecutive s. The number of trials, which are entries to the dark compartment, was recorded [35].
Retention test
In the retention test, the rats were placed in the light compartment for 5 s, and then the guillotine door was raised. The delay in the entrance to the dark compartment (i.e., staying in the light compartment) or Step-Through Latency in the retention trial (STLr) and the time spending in the Dark Compartment (TDC) was recorded up to 300 s. The retention test was finished when the animal did not enter the dark chamber within 300 s [36].
Morris Water Maze (MWM)
Evaluation of spatial working and reference memory was performed using MWM. Briefly, a large circular black pool (180 cm diameter and 60 cm height) filled to a depth of 35 cm with opaque water (22°C±1°C) was used. Non-motile external cues were located around the pool as a guide for rats to find their roots. The pool was divided into north (N), east (E), west (W), and south (S) quadrants. A hidden platform was placed in the center of the northern quadrant at 1 cm below the water surface. Rats were assessed on 5 consecutive days between 10:00 AM and 12:00 PM. The rats were allowed to do training that consisted of two blocks each includes 4 trials. Evaluation of visual test was carried out at day one by placing the clear covering platform on the water surface. The platform (with no cover) was placed at 0.5 cm below the water surface during trial days 2, 3, and 4 for checking short-term or working memory. On day 5 (probe stage), the platform was removed, and rats were allowed to swim for 60 s to evaluate long-term or reference memory. Videotaping of animal performance was performed using a video camera (Nikon, Melville, NY, USA) that was installed above the pool, and it was connected to a tracking system to record the animal’s performance. In the first 4 days of training, following placing in each of the quadrants, the rats were permitted to find the platform in 90 s. About 30 s rest was allocated between the two trials, and 5 min rest was allowed between the two blocks. Animals with no capability of finding the platform during each trial by themselves were manually placed on the platform. During the probe stage, all animals were placed in the pool from the western side. In these animals, recognition of the exact location of the place of platform was calculated by recording the percentage of time spent in the target quadrant [37].
Tissue preparation and Immunohistochemistry
After 60 days, the animals were deeply anesthetized with ketamine and xylazine, perfused transcardially with 150–200 mL PBS and fixed with 4% paraformaldehyde. After whole-brain primary fixation, the hippocampus was harvested and re-fixed with 4% paraformaldehyde. Specimens were embedded in paraffin (Merck, Germany), and 5-µm paraffin sections were prepared for immunohistochemistry evaluation. After deparaffinization and antigen retrieval, the sections were rinsed with PBS, treated with blocking solution, and incubated with anti-amyloid precursor protein antibody (ab49385. 1:2000, Abcam) primary antibodies overnight at 4°C. The next day, after washing with PBS, the sections were incubated with the secondary antibody: goat anti-rabbit (ab6721, Abcam) for 2 h at room temperature. After extensive washing in PBS, the sites of antibody binding were visualized using the avidin-biotin-peroxidase method (ABC Standard kit, VECTASTAIN, VectorLabs). Diaminobenzidine (DAB) was used as a chromogen. The sections were counterstained with hematoxylin and cover-slipped with entellan [38].
Statistical analysis
Homogeneity of variances (Levene’s test) was checked, and data revealed normal distribution (The Shapiro-Wilk’s test). All values were presented as mean±SD. One-way Analysis of Variance (ANOVA) using SPSS V. 22 (SPSS Inc., Chicago, IL) was applied for data analysis. MVM, STLr, and TDC showed unequal variances, thereby Tamhane used as a Post Hoc Test. ANOVA results for STLa and the number of trials was not significant. P≤0.05 was considered statistically significant.
Results
PAL test findings
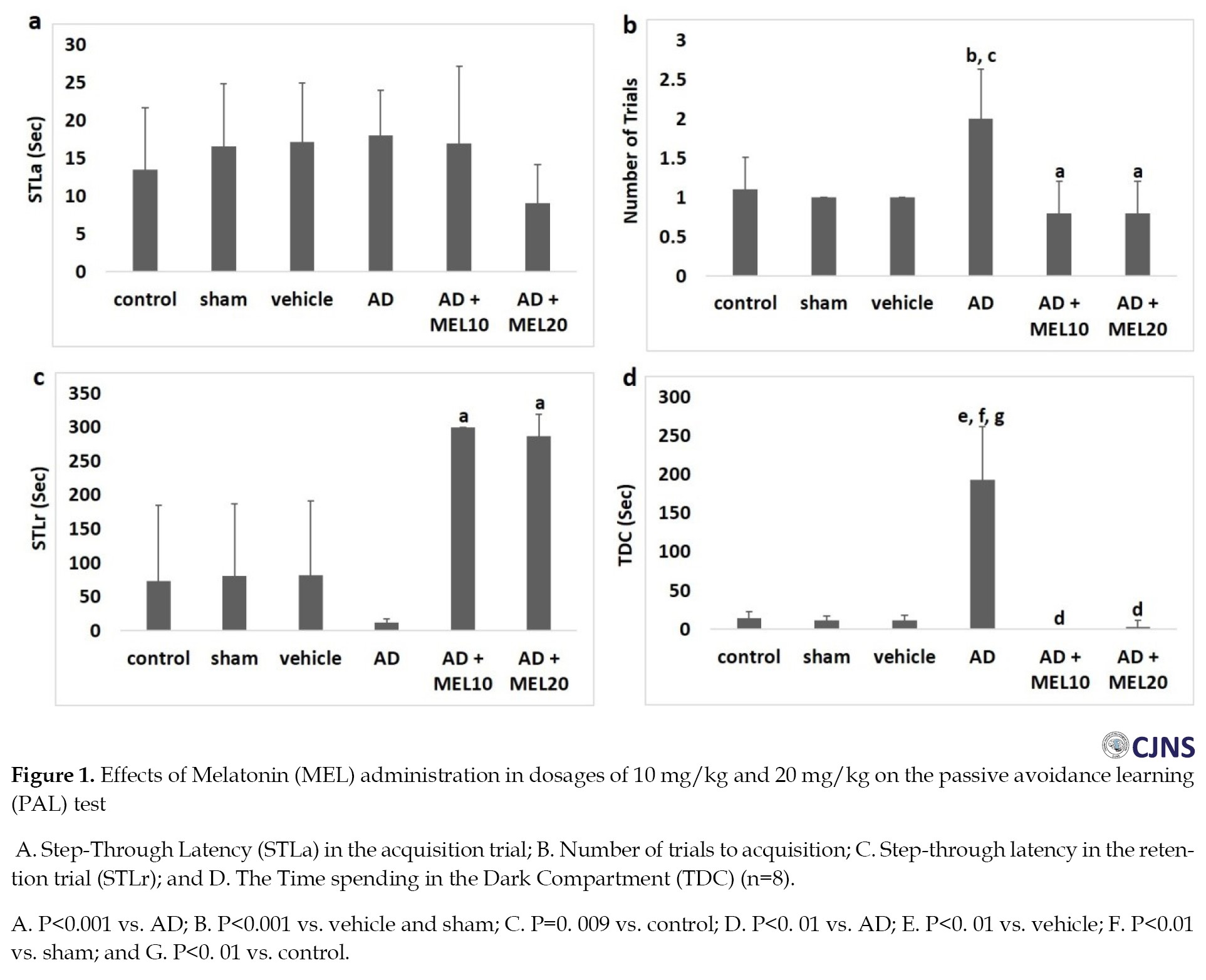
The STLa recording was performed in animals placing in the dark compartment without receiving an electric shock. Entrance latency to the darkness compartment showed no considerable difference (Figure 1a). The number of trials showed significant differences in the AD+MEL 10 and AD+MEL 20 groups compared with the AD group (P=0.000). However, there were no significant differences in the number of trials to acquisition between the AD+MEL10 and AD+EL20 groups (Figure 1b).
The STLr (Figure 1c) and TDC (Figure 1d) were also recorded in animals. In the retention test, MEL-treated groups showed a rise in the STLr and a fall in the TDC. The STLr and TDC for the AD+EL10 group were 300±0 s (P=0.000 vs. AD group) and 0±0 s (P=0.000 vs. AD group). The STLr and TDC for the AD+MEL20 group were 286.6±32.65 s (P=0.000 vs. AD group) and 2.8±8.1 s (P=0.000 vs. AD group), respectively. Both STLr and TDC showed no significant changes in the AD+MEL 20 group compared with the AD+MEL10 group.
MVM test results
The mean time was spent in the target quadrant was recorded in the probe trial stage. We found that the two MEL-treated groups spent less time in the target quadrant. One-way ANOVA showed a significant difference between experimental groups. The Mean±SD for AD+MEL10 and AD+MEL20 groups were 15.67±3.09 (P =0.000 vs. AD group) and 17.64±2.7 (P =0.000 vs. AD group), respectively. There were no significant changes in the AD+EL10 group compared with the AD+MEL 20 group (P>0.05) (Figure 2). Histological findings
Our finding indicated that the number of APP deposit was significantly decreased in the brain of both groups received melatonin (P=0.000). However, their numbers in the MEL 20 groups were less than in the MEL10 group (Figure 3). Discussion
In the present study, effects of MEL application at doses of 10 mg/kg and 20 mg/kg were evaluated in the Aβ-induced AD. Evaluations of learning and spatial memory, level of APP deposit was performed to target these effects.
Here, we found no changes in the learning of passive avoidance tests in all groups. The AD+MEL 10 and AD+MEL 20 groups showed a considerable fall in the number of acquisition trials compared to the AD group, possibly showing improvement in the acquisition.
TDC is considered as an indicator of inhibitory avoidance behavior [3]. In the retention test, we found that the AD group had a decrease in the STLr, a significant increase in the TDC, indicating Aβ-induced deficits of memory retention [36]. On the other hand, we found an increase of the STLr but a decrease of the TDC in both AD+MEL10 and AD+MEL 20 groups compared with the AD group. These results elucidate the possible facilitatory effects of both dosages of MEL on memory retention [36]. However, the results of both STLr and TDC tests showed no considerable differences in the AD+MEL20 group compared to the AD+MEL10 group, which means no possible difference in using the two dosages of MEL for targeting learning deficit in rats under exposure to Aβ.
MWM was also tested in the studying groups, and the results revealed that animals in the AD group spent less time in the target quadrant indicating spatial memory deficit induced by Aβ injection in this group. On the other hand, upon MEL treatment, the animals showed significantly more time spent in the target quadrant. The effect of MEL against scopolamine has been reported in rats [38]. The preventive role of MEL has been shown in rats who received an intrahippocampal injection of Aβ followed by an application of melatonin for 10 days [39]. This means that MEL administration can reverse memory impairment induced by Aβ [19, 20, 21], which was in line with the work performed by Rudnitskaya et al. on OXYS-injured rats that they found MEL-treated rats spent more time in the target quadrant, suggesting the positive effect of MEL at dosage 0.04 mg/kg on the improvement of memory deficit. In their study, they also noticed that oral administration of MEL could reduce the accumulation of Aβ in the hippocampus [14]. Similarly, Zhang et al. found that application of 500 mg/kg MEL at 24 h before Aβ1–42 injection could improve impairment of spatial learning and memory [40]. Here, we found beneficial effects of MEL dosages 10 mg/kg and 20 mg/kg spatial learning and memory after injection of the final dosage of the Aβ1–42.
There is evidence that Aβ can induce oxidative damage in mitochondrial DNA [5, 6, 8]. Sharif et al. assessed the effects of 50 and 100 µg/kg of MEL on H89-induced memory deficit, and they found that MEL, a powerful scavenger of ROS, exert its neuroprotection through reduction of oxidative stress [9, 15] and increases mitochondrial function [7, 18, 41]. The pineal hormone melatonin [10] stimulates the nonamyloidogenic processing and inhibits the amyloidogenic processing of β-amyloid precursor protein [22, 23]. Melatonin has a neuroprotective effect in the treatment of AD [20, 42]. We may have assumed in our study that protective roles of 10 mg/kg and 20 mg/kg of MEL against Aβ were probably applied through targeting oxidative stress. Our immunohistochemical findings demonstrate that the groups that received melatonin had the clearance ability of APP deposits, resulting in cognitive performance improving AD rat models. The present study is concurrent with another study indicating that melatonin protects neurons from the toxicity of the amyloid-β (Aβ) peptide (the main neurotoxin involved in AD) through GABA receptors [43]. Melatonin proteolytic cleavage of APP through the α-secretase pathway is controlled by many physiological and pathological stimuli, especially through Protein Kinase (PK) C and secretase-mediated cleavage APP [43, 44].
Interestingly, we found no significant difference between 10 mg/kg and 20 mg/kg of MEL on spatial memory. Considering this point along with the mentioned works by others, we speculate that lower doses of MEL administration could be neuroprotective against Aβ-induced memory deficit as much as the higher dosages of this hormone do, and possibly there is no need to use high doses aiming to exert better results [30]. Our findings showed that the application of dosages 10 mg/kg and 20 mg/kg of MEL did not have any significant difference in exerting protective roles against learning and spatial memory deficit.
Overall, the present study results showed no significant difference between melatonin doses (10 and 20 mg/kg) in the behavioral and histological assessment. Besides the dose, the duration of treatment with melatonin is also a factor that affects various studies of AD animal models. For example, a study indicated that melatonin injection (0.5 mg/d in drinking water) for 8–11 months significantly reduced cognitive deficits in Barnes maze and MWM and novel object recognition tests [45], while in another study, intraperitoneal injection of melatonin at a dose of 0.1, 1, and 10 mg/kg for 10 days induced spatial memory impairment [28]. Also, the results of another study showed that intraperitoneal injection of melatonin (30 mg/kg/d) for 10 days did not affect passive avoidance memory in AD rats [30].
One of our study limitations is that we only assessed the melatonin effect at doses of 10 and 20 mg/kg. Assessment of other doses of melatonin would be useful to understand the optimal dose of melatonin on the memory defect. Although it is cumbersome, we suggest the measuring of MWM, PAL, and histological assessment several times because it would be useful to understand the temporal profile of the melatonin effects on memory deficits.
Conclusion
Our findings declared that 10 and 20 mg/kg doses of melatonin have similar results on learning and memory in the AD model. But 20 mg/kg of melatonin has significantly more effect on the clearance of APP deposition.
Ethical Considerations
Compliance with ethical guidelines
The present study was approved by the Ethics Committee of Hamadan University of Medical Sciences (Code: IR.UMSHA.REC.1394.278). All study procedures were done in compliance with the ethical guidelines of the 2013 version of the Declaration of Helsinki.
Funding
This work was supported by Hamadan University of Medical Sciences (Grant no. 9407073723).
Authors contributions
Conceptualization: Arman Keymoradzadeh, Ali reza Komaki, Nafise Faraji, Zoleikha Golipoor; Methodology: Arman Keymoradzadeh, Arash Bakhshi, Nafise Faraji, Zoleikha Golipoor; Investigation, writing the original draft, review, and editing: All authors. Funding, acquisition: Zoleikha Golipoor; Resources: Arman Keymoradzadeh, Arash Bakhshi, Zoleikha Golipoor; Supervision: Arman Keymoradzadeh, Zoleikha Golipoor.
Conflict of interest
The authors declared no conflict of interest.
References
lzheimer disease (AD) is an age-related neurodegenerative disorder characterized by a progressive impairment of cognitive function [1, 2]. AD targets vital brain cells through which it affects thinking, memory, and behavior: these effects compromise work and social life [3].
Pathogenesis of AD has not been fully understood; however, it is evident that amyloid-βeta (Aβ) accumulation is an integral part of this disease [4], as it plays an essential role in the AD-related neurodegenerative process [3]. Aβ is a peptide containing 37–43 amino acids, and it is produced from Amyloid Precursor Protein (APP) [5] through a proteolytic cleavage mediated by β- and γ-secretases [4]. Intraventricular injection of Aβ is known to induce learning and memory deficits [3]. Toxic effects of Aβ in the brain may exert through an increase in neuroinflammation, mitochondrial dysfunction, and Reactive Oxygen Species (ROS), through which it induces oxidative stress in the brain [4, 6, 7, 8]. Oxidative stress plays a key role in learning and memory impairment [9].
Melatonin (MEL) (N-acetyl-5-methoxytryptamine) is a production of the pineal gland [10] and a multifunctional molecule that participate in a variety of physiological functions, including antioxidant [11, 12, 13, 14] and radical scavenger against harmful effects of free radicals on biological membrane lipids, Deoxyribonucleic Acid (DNA), and proteins [12 ,15]. Melatonin appears effective and safe in improving sleep quality in patients with AD [16]. There is growing evidence that sheds light on the protective role of MEL in memory deficit [9], aging, and Alzheimer disease [17]. MEL can be served as a neuroprotective hormone against mitochondrial damage [18], oxidative stress resulting in amelioration of learning deficits and neurodegenerative diseases [19, 20]. MEL has anti-amyloid properties through inhibition of Aβ generation [21]. Also, MEL stimulates the nonamyloidogenic pathway over the amyloidogenic pathway in the cultured neuronal and non-neuronal cells [22, 23, 24].
It has been suggested that melatonin has sufficient high concentrations in the cerebral tissue [25], and melatonin analogs are employed in higher densities [26]. However, different doses of melatonin have been shown to have different effects on memory in animal models of memory impairment; for example, one study indicated that melatonin at a dose of 10 mg/kg improves memory [27], while another study found no effect on memory [28]. In another study, a dose of 20 mg/kg melatonin improved memory [29], suggesting that doses greater than 10 mg/kg may improve memory, whereas, in their study, a dose of 30 mg did not affect memory [30]. Thus, this study aimed to evaluate the treatment role of two different MEL dosages on the learning and memory, Amyloid Precursor Protein (APP) deposit and migration of microglia in AD rats.
Materials and Methods
Animals
A total of 48 male Wistar rats weighing 250-300 g were obtained from the Medical Faculty of Hamedan University of Medical Sciences. The rats were placed in standard conditions of illumination (12 h light-dark cycle), humidity (55%-65%), and temperature (22°C). They were allowed to have easy access to food and water ad libitum for one week to be acclimatized to the new environment. All procedures were performed by reliance on the guides from the Ethics Committee of Hamedan University of Medical Sciences compliance to the institutional and national guidelines for animal care and use.
Experimental design
Animals were divided randomly into 6 groups, including control, sham, vehicle, AD, AD+MEL10, and AD+MEL 20. AD received Aβ1-42 on the first day of experiments. After two weeks, MEL (Sigma) at two doses of 10 mg/kg and 20 mg/kg was administered intraperitoneally once per week for 4 weeks [28, 29]. Then, the learning and spatial memory of the rats were evaluated. Morris Water Maze (MWM) and Passive Avoidance Learning (PAL) tests were used to assess memory function. Also, APP microglia was assessed by histological study, respectively.
Surgery and injection of Aβ (1–42)
The procedure was done according to previous studies [31, 32]. The animals were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and submitted to a stereotaxic apparatus (Stoelting, USA). According to the bregma, the coordinates for the bilateral intracerebroventricular were AP: 1.2 mm; ML: 2 mm; and DV: 4 mm [33]. Aβ (Tocris Bioscience, UK) solution (1 µg/µL, 5 μL) was bilaterally microinjected into the region. Sham rats only sustained surgery procedure without any injection. Vehicle groups received the same volume of vehicle. Following injections, the skin was sutured, and the animals were allowed to recover in their home cages.
Passive Avoidance Learning (PAL)
Apparatus
PAL was evaluated using a step-through apparatus [34] consisted of two compartments: a light compartment (20 cm × 20 cm × 30 cm) made from transparent plastic and a dark compartment made from dark opaque plastic (20 cm × 20 cm × 30 cm). A stainless steel rod with a diameter of 3 mm was applied to the floor of the compartments. A shock generator (Behbood Pardaz Co. Tehran, Iran) was used to electrify the dark compartment floor. A rectangular opening (6 cm × 8 cm) capable of closing by an opaque guillotine door was located between the two compartments [34].
Training
First, each rat received two trials to become familiarized with the apparatus. For this purpose, the rats were located in the light compartment facing away from the door for 5 s. Then, the guillotine door was raised. Typically, the rats have an instinctive tendency to the dark environment. When the rats entered the dark compartment, the guillotine door was closed, and the animals were kept for 30 s in this compartment. This trial was repeated after 30 min interval. The entrance latency to the dark compartment (STLa: Step-through latency in the acquisition) was tested when the rats were placed in the dark compartment [35].
After the rats entered the dark compartment spontaneously, the guillotine door was closed for 30 s, and a 0.5 mA electrical shock was applied for 3 s. The procedure was repeated after 2 min. The training was finished when animals stayed in the light compartment for 120 consecutive s. The number of trials, which are entries to the dark compartment, was recorded [35].
Retention test
In the retention test, the rats were placed in the light compartment for 5 s, and then the guillotine door was raised. The delay in the entrance to the dark compartment (i.e., staying in the light compartment) or Step-Through Latency in the retention trial (STLr) and the time spending in the Dark Compartment (TDC) was recorded up to 300 s. The retention test was finished when the animal did not enter the dark chamber within 300 s [36].
Morris Water Maze (MWM)
Evaluation of spatial working and reference memory was performed using MWM. Briefly, a large circular black pool (180 cm diameter and 60 cm height) filled to a depth of 35 cm with opaque water (22°C±1°C) was used. Non-motile external cues were located around the pool as a guide for rats to find their roots. The pool was divided into north (N), east (E), west (W), and south (S) quadrants. A hidden platform was placed in the center of the northern quadrant at 1 cm below the water surface. Rats were assessed on 5 consecutive days between 10:00 AM and 12:00 PM. The rats were allowed to do training that consisted of two blocks each includes 4 trials. Evaluation of visual test was carried out at day one by placing the clear covering platform on the water surface. The platform (with no cover) was placed at 0.5 cm below the water surface during trial days 2, 3, and 4 for checking short-term or working memory. On day 5 (probe stage), the platform was removed, and rats were allowed to swim for 60 s to evaluate long-term or reference memory. Videotaping of animal performance was performed using a video camera (Nikon, Melville, NY, USA) that was installed above the pool, and it was connected to a tracking system to record the animal’s performance. In the first 4 days of training, following placing in each of the quadrants, the rats were permitted to find the platform in 90 s. About 30 s rest was allocated between the two trials, and 5 min rest was allowed between the two blocks. Animals with no capability of finding the platform during each trial by themselves were manually placed on the platform. During the probe stage, all animals were placed in the pool from the western side. In these animals, recognition of the exact location of the place of platform was calculated by recording the percentage of time spent in the target quadrant [37].
Tissue preparation and Immunohistochemistry
After 60 days, the animals were deeply anesthetized with ketamine and xylazine, perfused transcardially with 150–200 mL PBS and fixed with 4% paraformaldehyde. After whole-brain primary fixation, the hippocampus was harvested and re-fixed with 4% paraformaldehyde. Specimens were embedded in paraffin (Merck, Germany), and 5-µm paraffin sections were prepared for immunohistochemistry evaluation. After deparaffinization and antigen retrieval, the sections were rinsed with PBS, treated with blocking solution, and incubated with anti-amyloid precursor protein antibody (ab49385. 1:2000, Abcam) primary antibodies overnight at 4°C. The next day, after washing with PBS, the sections were incubated with the secondary antibody: goat anti-rabbit (ab6721, Abcam) for 2 h at room temperature. After extensive washing in PBS, the sites of antibody binding were visualized using the avidin-biotin-peroxidase method (ABC Standard kit, VECTASTAIN, VectorLabs). Diaminobenzidine (DAB) was used as a chromogen. The sections were counterstained with hematoxylin and cover-slipped with entellan [38].
Statistical analysis
Homogeneity of variances (Levene’s test) was checked, and data revealed normal distribution (The Shapiro-Wilk’s test). All values were presented as mean±SD. One-way Analysis of Variance (ANOVA) using SPSS V. 22 (SPSS Inc., Chicago, IL) was applied for data analysis. MVM, STLr, and TDC showed unequal variances, thereby Tamhane used as a Post Hoc Test. ANOVA results for STLa and the number of trials was not significant. P≤0.05 was considered statistically significant.
Results
PAL test findings
The STLa recording was performed in animals placing in the dark compartment without receiving an electric shock. Entrance latency to the darkness compartment showed no considerable difference (Figure 1a). The number of trials showed significant differences in the AD+MEL 10 and AD+MEL 20 groups compared with the AD group (P=0.000). However, there were no significant differences in the number of trials to acquisition between the AD+MEL10 and AD+EL20 groups (Figure 1b).
The STLr (Figure 1c) and TDC (Figure 1d) were also recorded in animals. In the retention test, MEL-treated groups showed a rise in the STLr and a fall in the TDC. The STLr and TDC for the AD+EL10 group were 300±0 s (P=0.000 vs. AD group) and 0±0 s (P=0.000 vs. AD group). The STLr and TDC for the AD+MEL20 group were 286.6±32.65 s (P=0.000 vs. AD group) and 2.8±8.1 s (P=0.000 vs. AD group), respectively. Both STLr and TDC showed no significant changes in the AD+MEL 20 group compared with the AD+MEL10 group.
MVM test results
The mean time was spent in the target quadrant was recorded in the probe trial stage. We found that the two MEL-treated groups spent less time in the target quadrant. One-way ANOVA showed a significant difference between experimental groups. The Mean±SD for AD+MEL10 and AD+MEL20 groups were 15.67±3.09 (P =0.000 vs. AD group) and 17.64±2.7 (P =0.000 vs. AD group), respectively. There were no significant changes in the AD+EL10 group compared with the AD+MEL 20 group (P>0.05) (Figure 2). Histological findings
Our finding indicated that the number of APP deposit was significantly decreased in the brain of both groups received melatonin (P=0.000). However, their numbers in the MEL 20 groups were less than in the MEL10 group (Figure 3). Discussion
In the present study, effects of MEL application at doses of 10 mg/kg and 20 mg/kg were evaluated in the Aβ-induced AD. Evaluations of learning and spatial memory, level of APP deposit was performed to target these effects.
Here, we found no changes in the learning of passive avoidance tests in all groups. The AD+MEL 10 and AD+MEL 20 groups showed a considerable fall in the number of acquisition trials compared to the AD group, possibly showing improvement in the acquisition.
TDC is considered as an indicator of inhibitory avoidance behavior [3]. In the retention test, we found that the AD group had a decrease in the STLr, a significant increase in the TDC, indicating Aβ-induced deficits of memory retention [36]. On the other hand, we found an increase of the STLr but a decrease of the TDC in both AD+MEL10 and AD+MEL 20 groups compared with the AD group. These results elucidate the possible facilitatory effects of both dosages of MEL on memory retention [36]. However, the results of both STLr and TDC tests showed no considerable differences in the AD+MEL20 group compared to the AD+MEL10 group, which means no possible difference in using the two dosages of MEL for targeting learning deficit in rats under exposure to Aβ.
MWM was also tested in the studying groups, and the results revealed that animals in the AD group spent less time in the target quadrant indicating spatial memory deficit induced by Aβ injection in this group. On the other hand, upon MEL treatment, the animals showed significantly more time spent in the target quadrant. The effect of MEL against scopolamine has been reported in rats [38]. The preventive role of MEL has been shown in rats who received an intrahippocampal injection of Aβ followed by an application of melatonin for 10 days [39]. This means that MEL administration can reverse memory impairment induced by Aβ [19, 20, 21], which was in line with the work performed by Rudnitskaya et al. on OXYS-injured rats that they found MEL-treated rats spent more time in the target quadrant, suggesting the positive effect of MEL at dosage 0.04 mg/kg on the improvement of memory deficit. In their study, they also noticed that oral administration of MEL could reduce the accumulation of Aβ in the hippocampus [14]. Similarly, Zhang et al. found that application of 500 mg/kg MEL at 24 h before Aβ1–42 injection could improve impairment of spatial learning and memory [40]. Here, we found beneficial effects of MEL dosages 10 mg/kg and 20 mg/kg spatial learning and memory after injection of the final dosage of the Aβ1–42.
There is evidence that Aβ can induce oxidative damage in mitochondrial DNA [5, 6, 8]. Sharif et al. assessed the effects of 50 and 100 µg/kg of MEL on H89-induced memory deficit, and they found that MEL, a powerful scavenger of ROS, exert its neuroprotection through reduction of oxidative stress [9, 15] and increases mitochondrial function [7, 18, 41]. The pineal hormone melatonin [10] stimulates the nonamyloidogenic processing and inhibits the amyloidogenic processing of β-amyloid precursor protein [22, 23]. Melatonin has a neuroprotective effect in the treatment of AD [20, 42]. We may have assumed in our study that protective roles of 10 mg/kg and 20 mg/kg of MEL against Aβ were probably applied through targeting oxidative stress. Our immunohistochemical findings demonstrate that the groups that received melatonin had the clearance ability of APP deposits, resulting in cognitive performance improving AD rat models. The present study is concurrent with another study indicating that melatonin protects neurons from the toxicity of the amyloid-β (Aβ) peptide (the main neurotoxin involved in AD) through GABA receptors [43]. Melatonin proteolytic cleavage of APP through the α-secretase pathway is controlled by many physiological and pathological stimuli, especially through Protein Kinase (PK) C and secretase-mediated cleavage APP [43, 44].
Interestingly, we found no significant difference between 10 mg/kg and 20 mg/kg of MEL on spatial memory. Considering this point along with the mentioned works by others, we speculate that lower doses of MEL administration could be neuroprotective against Aβ-induced memory deficit as much as the higher dosages of this hormone do, and possibly there is no need to use high doses aiming to exert better results [30]. Our findings showed that the application of dosages 10 mg/kg and 20 mg/kg of MEL did not have any significant difference in exerting protective roles against learning and spatial memory deficit.
Overall, the present study results showed no significant difference between melatonin doses (10 and 20 mg/kg) in the behavioral and histological assessment. Besides the dose, the duration of treatment with melatonin is also a factor that affects various studies of AD animal models. For example, a study indicated that melatonin injection (0.5 mg/d in drinking water) for 8–11 months significantly reduced cognitive deficits in Barnes maze and MWM and novel object recognition tests [45], while in another study, intraperitoneal injection of melatonin at a dose of 0.1, 1, and 10 mg/kg for 10 days induced spatial memory impairment [28]. Also, the results of another study showed that intraperitoneal injection of melatonin (30 mg/kg/d) for 10 days did not affect passive avoidance memory in AD rats [30].
One of our study limitations is that we only assessed the melatonin effect at doses of 10 and 20 mg/kg. Assessment of other doses of melatonin would be useful to understand the optimal dose of melatonin on the memory defect. Although it is cumbersome, we suggest the measuring of MWM, PAL, and histological assessment several times because it would be useful to understand the temporal profile of the melatonin effects on memory deficits.
Conclusion
Our findings declared that 10 and 20 mg/kg doses of melatonin have similar results on learning and memory in the AD model. But 20 mg/kg of melatonin has significantly more effect on the clearance of APP deposition.
Ethical Considerations
Compliance with ethical guidelines
The present study was approved by the Ethics Committee of Hamadan University of Medical Sciences (Code: IR.UMSHA.REC.1394.278). All study procedures were done in compliance with the ethical guidelines of the 2013 version of the Declaration of Helsinki.
Funding
This work was supported by Hamadan University of Medical Sciences (Grant no. 9407073723).
Authors contributions
Conceptualization: Arman Keymoradzadeh, Ali reza Komaki, Nafise Faraji, Zoleikha Golipoor; Methodology: Arman Keymoradzadeh, Arash Bakhshi, Nafise Faraji, Zoleikha Golipoor; Investigation, writing the original draft, review, and editing: All authors. Funding, acquisition: Zoleikha Golipoor; Resources: Arman Keymoradzadeh, Arash Bakhshi, Zoleikha Golipoor; Supervision: Arman Keymoradzadeh, Zoleikha Golipoor.
Conflict of interest
The authors declared no conflict of interest.
References
- Shahidi S, Zargooshnia S, Asl SS, Komaki A, Sarihi A. Influence of N-acetyl cysteine on beta-amyloid-induced Alzheimer’s disease in a rat model: A behavioral and electrophysiological study. Brain Res Bull. 2017; 131:142-9. [DOI:10.1016/j.brainresbull.2017.04.001] [PMID]
- Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010; 362:329-44. [DOI:10.1056/NEJMra0909142] [PMID]
- Asadbegi M, Yaghmaei P, Salehi I, Komaki A, Ebrahim-Habibi A. Investigation of thymol effect on learning and memory impairment induced by intrahippocampal injection of amyloid beta peptide in high fat diet-fed rats. Metab Brain Dis. 2017; 32(3):827-39. [DOI:10.1007/s11011-017-9960-0] [PMID]
- Nell HJ, Au JL, Giordano CR, Terlecky SR, Walton PA, Whitehead SN, et al. Targeted antioxidant, catalase-SKL, reduces beta‐amyloid toxicity in the rat brain. Brain Pathol. 2017; 27(1):86-94. [DOI:10.1111/bpa.12368] [PMID]
- Bozner P, Grishko V, LeDoux SP, Wilson GL, Chyan YC, Pappolla MA. The amyloid β protein induces oxidative damage of mitochondrial DNA. J Neuropathol Exp Neurol. 1997; 56(12):1356-62. [DOI:10.1097/00005072-199712000-00010] [PMID]
- Hsu MJ, Sheu JR, Lin CH, Shen MY, Hsu CY. Mitochondrial mechanisms in amyloid beta peptide-induced cerebrovascular degeneration. Biochim Biophys Acta. 2010; 1800(3):290-6. [DOI:10.1016/j.bbagen.2009.08.003] [PMID]
- Cardinali DP, Pagano ES, Bernasconi PA, Reynoso R, Scacchi P. Melatonin and mitochondrial dysfunction in the central nervous system. Horm behave. 2013; 63(2):322-30. [DOI:10.1016/j.yhbeh.2012.02.020] [PMID]
- Babaei Abraki S, Chavoshi-Nezhad S. [Mitochondrial defects and oxidative stress in Alzheimer Disease (Persian)]. Shefaye Khatam. 2014; 2(1):85-94. [DOI:10.18869/acadpub.shefa.2.1.85]
- Sharif R, Aghsami M, Gharghabi M, Sanati M, Khorshidahmad T, Vakilzadeh G, et al. Melatonin reverses H-89 induced spatial memory deficit: Involvement of oxidative stress and mitochondrial function. Behav Brain Res. 2017; 316:115-24. [DOI:10.1016/j.bbr.2016.08.040] [PMID]
- Arendt J. Melatonin and the pineal gland: influence on mammalian seasonal and circadian physiology. Rev Reprod. 1998; 3(1):13-22. [DOI:10.1530/ror.0.0030013] [PMID]
- Mortezaee K, Sabbaghziarani F, Omidi A, Dehpour AR, Omidi N, Ghasemi S, et al. Therapeutic value of melatonin post-treatment on CCl4-induced fibrotic rat liver. Can J Physiol Pharmacol. 2015; 94(2):119-30. [DOI:10.1139/cjpp-2015-0266] [PMID]
- Mortezaee K, Pasbakhsh P, Kashani IR, Sabbaghziarani F, Omidi A, Zendedel A, et al. Melatonin pretreatment enhances the homing of bone marrow-derived mesenchymal stem cells following transplantation in a rat model of liver fibrosis. Iran Biomed J. 2016; 20(4):207-16. [PMCID] [PMID]
- Mortezaee K, Khanlarkhani N, Sabbaghziarani F, Nekoonam S, Majidpoor J, Hosseini A, et al. Preconditioning with melatonin improves therapeutic outcomes of bone marrow-derived mesenchymal stem cells in targeting liver fibrosis induced by CCl4. Cell Tissue Res. 2017; 369(2):303-12. [DOI:10.1007/s00441-017-2604-1] [PMID]
- Rudnitskaya EA, Muraleva NA, Maksimova KY, Kiseleva E, Kolosova NG, Stefanova NA. Melatonin attenuates memory impairment, amyloid-β accumulation, and neurodegeneration in a rat model of sporadic Alzheimer’s disease. J Alzheimers Dis. 2015; 47(1):103-16. [DOI:10.3233/JAD-150161] [PMID]
- Acuna Castroviejo D, C Lopez L, Escames G, López A, A Garcia J, J Reiter R. Melatonin-mitochondria interplay in health and disease. Curr Top Med Chem. 2011; 11(2):221-40. [DOI:10.2174/156802611794863517] [PMID]
- Wang YY, Zheng W, Ng CH, Ungvari GS, Wei W, Xiang YT. Meta-analysis of randomized, double-blind, placebo-controlled trials of melatonin in Alzheimer’s disease. Int J Geriatr Psychiatry. 2017; 32(1):50-7. [DOI:10.1002/gps.4571] [PMID]
- Wu YH, Swaab DF. The human pineal gland and melatonin in aging and Alzheimer’s disease. J Pineal Res. 2005; 38(3):145-52. [DOI:10.1111/j.1600-079X.2004.00196.x] [PMID]
- Mendivil-Perez M, Soto-Mercado V, Guerra-Librero A, Fernandez-Gil BI, Florido J, Shen YQ, et al. Melatonin enhances neural stem cell differentiation and engraftment by increasing mitochondrial function. J Pineal Res. 2017; 63(2):e12415. [DOI:10.1111/jpi.12415] [PMID]
- Srinivasan V. Melatonin oxidative stress and neurodegenerative diseases. Indian J Exp Biol. 2002; 40(6):668-79. http://nopr.niscair.res.in/bitstream/123456789/23510/1/IJEB%2040%286%29%20668-679.pdf
- Polimeni G, Esposito E, Bevelacqua V, Guarneri C, Cuzzocrea S. Role of melatonin supplementation in neurodegenerative disorders. Front Biosci (Landmark Ed). 2014; 19:429-46. [DOI:10.2741/4217] [PMID]
- Mukda S, Panmanee J, Boontem P, Govitrapong P. Melatonin administration reverses the alteration of amyloid precursor protein-cleaving secretases expression in aged mouse hippocampus. Neurosci Lett. 2016; 621:39-46. [DOI:10.1016/j.neulet.2016.04.013] [PMID]
- Panmanee J, Nopparat C, Chavanich N, Shukla M, Mukda S, Song W, et al. Melatonin regulates the transcription of betaAPP-cleaving secretases mediated through melatonin receptors in human neuroblastoma SH-SY5Y cells. J Pineal Res. 2015; 59(3):308-20. [DOI:10.1111/jpi.12260] [PMID]
- Shukla M, Htoo HH, Wintachai P, Hernandez JF, Dubois C, Postina R, et al. Melatonin stimulates the nonamyloidogenic processing of betaAPP through the positive transcriptional regulation of ADAM10 and ADAM17. J Pineal Res. 2015; 58(2):151-65. [DOI:10.1111/jpi.12200] [PMID]
- Ghasemi Hamidabadi H, Rezvani Z, Nazm Bojnordi M, Shirinzadeh H, Seifalian AM, Joghataei MT, et al. Chitosan-Intercalated Montmorillonite/Poly (vinyl alcohol) Nanofibers as a platform to guide neuronlike differentiation of human dental pulp stem cells. ACS Appl Mater Interfaces. 2017; 9(13):11392-404. [DOI:10.1021/acsami.6b14283] [PMID]
- Legros C, Chesneau D, Boutin JA, Barc C, Malpaux B. Melatonin from cerebrospinal fluid but not from blood reaches sheep cerebral tissues under physiological conditions. J Neuroendocrinol 2014; 26(3):151-63. [DOI:10.1111/jne.12134] [PMID]
- Cardinali DP, Srinivasan V, Brzezinski A, Brown GM. Melatonin and its analogs in insomnia and depression. J Pineal Res. 2012; 52(4):365-75. [DOI:10.1111/j.1600-079X.2011.00962.x] [PMID]
- Peng C. Melatonin rescues synaptic/memory impairment by regulating the levels of c‐fos in tg2576 mice. Alzheimer Dement . 2015; 11(7S_Part_10):P495. [DOI:10.1016/j.jalz.2015.06.571]
- Soto-Moyano R, Burgos H, Flores F, Valladares L, Sierralta W, Fernández V, et al. Melatonin administration impairs visuo-spatial performance and inhibits neocortical long-term potentiation in rats. Pharmacol Biochem Behav. 2006; 85(2):408-14. [DOI:10.1016/j.pbb.2006.09.009] [PMID]
- Sharma M, Briyal S, Gupta Y. Effect of alpha lipoic acid, melatonin and trans resveratrol on intracerebroventricular streptozotocin induced spatial memory deficit in rats. Indian J Physiol Pharmacol. 2005; 49(4):395-402. https://www.researchgate.net/profile/Seema-Briyal/publication/7200982_
- Eslamizade MJ, Madjd Z, Rasoolijazi H, Saffarzadeh F, Pirhajati V, Aligholi H, et al. Impaired memory and evidence of histopathology in CA1 pyramidal neurons through injection of Aβ1-42 peptides into the frontal cortices of rat. Basic Clin Neurosci. 2016; 7(1):31-41. [PMCID] [PMID]
- Nikkhah A, Ghahremanitamadon F, Zargooshnia S, Shahidi S, Soleimani AS. Effect of amyloid β- peptide on passive avoidance learning in rats: A behavioral study. Avicenna J Neuro Psych Physio 2014; 1(1):e18664. [DOI:10.17795/ajnpp-18664]
- Shahidi S, Zargooshnia S, Asl SS, Komaki A, Sarihi A. Influence of N-acetyl cysteine on beta-amyloid-induced Alzheimer’s disease in a rat model: A behavioral and electrophysiological study. Brain Res Bull. 2017; 131:142-9. [DOI:10.1016/j.brainresbull.2017.04.001] [PMID]
- Cetin F, Yazihan N, Dincer S, Akbulut G. The effect of intracerebroventricular injection of beta amyloid peptide (1-42) on caspase-3 activity, lipid peroxidation, nitric oxide and NOS expression in young adult and aged rat brain. Turk Neurosurg. 2013; 23(2):144-50. [DOI:10.5137/1019-5149.JTN.5855-12.1]
- Kohara Y, Kuwahara R, Kawaguchi S, Jojima T, Yamashita K. Perinatal exposure to genistein, a soy phytoestrogen, improves spatial learning and memory but impairs passive avoidance learning and memory in offspring. Physiol Behav. 2014; 130:40-6. [DOI:10.1016/j.physbeh.2014.03.006] [PMID]
- Feng Y, Zhang LX, Chao DM. [Role of melatonin in spatial learning and memory in rats and its mechanism (Chinese)]. Sheng li xue bao: [Acta physiologica Sinica]. 2002; 54(1):65-70. [PMID]
- Hasanein P, Shahidi S. Effects of hypericum perforatum extract on diabetes‐induced learning and memory impairment in rats. Phytother Res. 2011; 25(4):544-9. [DOI:10.1002/ptr.3298] [PMID]
- Nasiri E, Alizadeh A, Roushandeh AM, Gazor R, Hashemi-Firouzi N, Golipoor Z. Melatonin-pretreated adipose-derived mesenchymal stem cells efficeintly improved learning, memory, and cognition in an animal model of Alzheimer’s disease. Metab Brain Dis. 2019; 34(4):1131-43. [DOI:10.1007/s11011-019-00421-4] [PMID]
- Alizadeh R, Hassanzadeh G, Soleimani M, taghi Joghataei M, Siavashi V, Khorgami Z, et al. Gender and age related changes in number of dopaminergic neurons in adult human olfactory bulb. J Chem Neuroanat. 2015; 69:1-6. [DOI:10.1016/j.jchemneu.2015.07.003] [PMID]
- Liu XJ, Yuan L, Yang D, Han WN, Li QS, Yang W, et al. Melatonin protects against amyloid‐β‐induced impairments of hippocampal LTP and spatial learning in rats. Synapse. 2013; 67(9):626-36. [DOI:10.1002/syn.21677] [PMID]
- Zhang S, Wang P, Ren L, Hu C, Bi J. Protective effect of melatonin on soluble Aβ 1-42-induced memory impairment, astrogliosis, and synaptic dysfunction via the Musashi1/Notch1/Hes1 signaling pathway in the rat hippocampus. Alzheimers Res Ther. 2016; 8(1):40. [DOI:10.1186/s13195-016-0206-x] [PMID] [PMCID]
- García JA, Ortiz F, Miana J, Doerrier C, Fernández-Ortiz M, Rusanova I, et al. Contribution of inducible and neuronal nitric oxide synthases to mitochondrial damage and melatonin rescue in LPS-treated mice. J Physiol Biochem. 2017; 73(2):235-44. [DOI:10.1007/s13105-017-0548-2] [PMID]
- Pandi-Perumal SR, BaHammam AS, Brown GM, Spence DW, Bharti VK, Kaur C, et al. Melatonin antioxidative defense: therapeutical implications for aging and neurodegenerative processes. Neurotox Res. 2013; 23(3):267-300. [DOI:10.1007/s12640-012-9337-4] [PMID]
- Cardinali DP, Vigo DE, Olivar N, Vidal MF, Brusco LI. Melatonin therapy in patients with Alzheimer’s disease. Antioxidants 2014; 3(2):245-77. [DOI:10.3390/antiox3020245] [PMID] [PMCID]
- Donnelly PS, Caragounis A, Du T, Laughton KM, Volitakis I, Cherny RA, et al. Selective intracellular release of copper and zinc ions from bis (thiosemicarbazonato) complexes reduces levels of Alzheimer disease amyloid-β peptide. J Biol Chem. 2008; 283(8):4568-77. [DOI:10.1074/jbc.M705957200] [PMID]
- O’Neal-Moffitt G, Delic V, Bradshaw PC, Olcese J. Prophylactic melatonin significantly reduces Alzheimer’s neuropathology and associated cognitive deficits independent of antioxidant pathways in AβPP swe/PS1 mice. Mol Neurodegener. 2015; 10(1):27. [DOI:10.1186/s13024-015-0027-6] [PMID] [PMCID]
Type of Study: Research |
Subject:
Special
Received: 2021/03/14 | Accepted: 2021/01/21 | Published: 2021/01/21
Received: 2021/03/14 | Accepted: 2021/01/21 | Published: 2021/01/21
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |