BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
URL: http://cjns.gums.ac.ir/article-1-308-en.html


 , Amirreza Ghayeghran *
, Amirreza Ghayeghran * 

 2, Hamidreza Hatamian3
2, Hamidreza Hatamian3 

 , Mozaffar Hosseini-Nejad4
, Mozaffar Hosseini-Nejad4 

 , Babak Bakhshayesh Eghbali4
, Babak Bakhshayesh Eghbali4 


2- Department of Neurology, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran. , gham1346@gmail.com
3- Department of Neurology, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran.
4- Department of Neurology, Neuroscience Research Center, Poursina Hospital, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
Introduction
The worst 21st century pandemic of a novel coronavirus emerged in Wuhan City, China, in Winter of 2019 ; then, it has rapidly spread throughout the world. Its primary manifestation is severe acute respiratory distress, i.e. [Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)] is named by the World Health Organization as Coronavirus Disease 2019 (COVID-19). At the beginning of April 2020, a million confirmed cases and > 60000 deaths have occurred to this condition around the world. Besides, the respiratory and systemic symptoms which are most frequently presented in COVID-19, some neurological symptoms have been reported [1].
Previous studies have indicated that beta coronaviruses, including SARS-CoV and Middle East Respiratory Syndrome-CoV (MERS-CoV), could attack the Central Nervous System (CNS) in human patients and experimental animals. SARS-CoV-2 shares a highly homologous sequence with these family of coronaviruses; accordingly, it is highly suspected that it possesses similar neuroinvasive and neurotrophic properties [2, 3].
Some evidence suggests that the virus’s neurotropism property results from the strong expression of the Angiotensin-Converting Enzyme 2 (ACE 2) target in the CNS [4].
Approximately 36% of infected patients have been reported with neurological symptoms, including dizziness, headache, ataxia, seizures, anosmia, ageusia, neuralgia, fatigue, twitching, and myalgia. These symptoms are more frequent in patients with severe COVID-19 and lower lymphocyte count [1].
Some researchers found the most conclusive evidence for the neurovirulence of COVID-19 by the isolation of COVID-19 Ribonucleic Acid (RNA) in the Cerebrospinal Fluid (CSF) [5].
Poyiadji reported the first case of COVID-19 with acute hemorrhagic necrotizing encephalopathy. A female who worked in airline presented with a 3-day history of fever, cough, and confusional state [6].
Furthermore, Jingyuan Liu diagnosed a COVID-19 patient with viral encephalitis in Beijing Ditan Hospital. The presence of SARS-CoV-2 in cerebrospinal fluid was confirmed by PCR in this patient [2].
In human patients and experimental animals, the brainstem is reported to be infected by SARS‐CoV. Some researchers suggest that the respiratory symptoms may be due to the involvement of the medullary cardiorespiratory center. Considering the high similarity between SARS‐CoV and SARS‐CoV2, brainstem involvement may occur by the latter, too [7, 8].
Kang Zhao et al. were the first group to report a case of acute myelitis in a COVID-19 patient in Wuhan City, China. The patient was a man who developed flaccid paraparesis limbs with urinary and bowel incontinence after a high fever (40° C), With a diagnosis of post-infectious acute myelitis and comprehensive treatment, the paralysis of bilateral lower limbs improved [9].
We reported another case of acute myelitis in a COVID-19 infected patient in the north of Iran during the pandemic of COVID-19.
Case Presentation
A 60-year-old patient with a history of diabetes mellitus, hyperlipidemia, and hypertension was referred to an academic hospital in northern Iran on March 8, 2019. Furthermore, he presented progressive weakness of lower limbs accompanied by urinary incontinence and constipation from three days before admission. He had developed fever, nausea, and vomiting about two weeks before admission.
At the time of referral, his gastrointestinal symptoms and fever had obviated. Vital signs were stable, as follows: O2 saturation: >95% at rest; temperature: 37° C; pulse rate: 80 beats/min; respiratory rate: 18 breaths/min, and Blood Pressure (BP): 120/70 mm Hg.
In the examination, the strength of the upper limbs was equal to the 5/5 Medical Research Council Scale (MRC) in the distal and proximal parts, and the strength of the lower limbs was 5/5 and 3/5 MRC in the distal and proximal parts, respectively. At this time, the Deep Tendon Reflexes (DTR) of the lower limb was measured as zero, which increased to 3+ during hospitalization, and the same value for the upper limb was 2+. Plantar reflex was upward. Hoffman was negative. The cranial nerves and finger to nose were normal, and heel to shin could not be performed because of weakness.
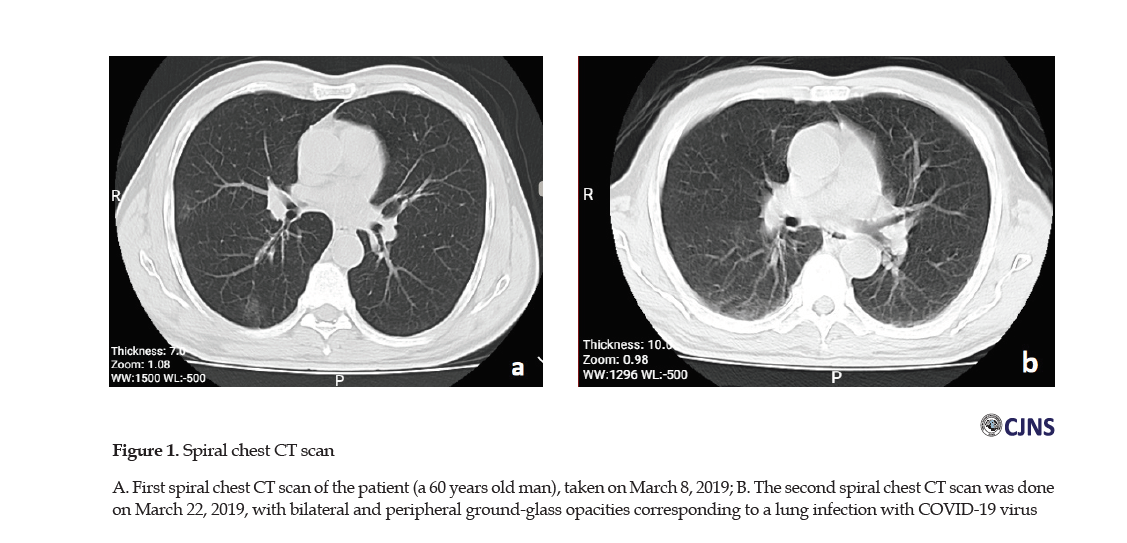
The patient’s drug history included Aspirin, metformin 500 mgBD, valsartan 80 mg/d, amlodipine 2.5 mg/d, and atorvastatin 40 mg/d. During his hospitalization, we started Clexane 60 daily for the prevention of Deep Vein Thrombosis (DVT), and due to the findings of his spiral chest Computerized Tomography (CT) scan (taken on March 8, 2019) corresponding to a lung infection with COVID-19 virus (bilateral and peripheral ground-glass opacities) (Figure 1A).
Moreover, we prescribed him with hydroxychloroquine 400 mg/d and oseltamivir 75mg, BID (twice a day) for 5 days. The post-admission laboratory findings of the patient are presented in Table 1.
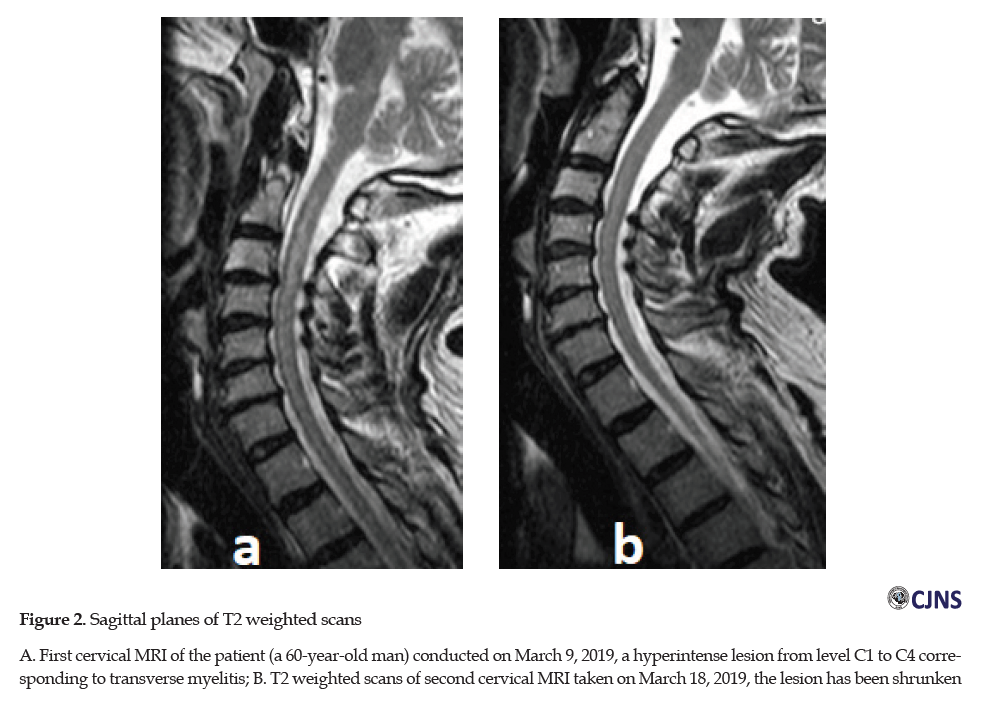
The electrodiagnostic study by Electromyography and Nerve Conduction Velocity (EMG-NCV) was normal. No lesion was found in the Magnetic Resonance Imaging ( MRI) of the thoracic and lumbar spinal cord. In the axial and sagittal planes of T2 weighted scans of cervical MRI performed on March 9, 2019, a hyperintense lesion from level C1 to C4 with mainly central involvement corresponding with cervical myelitis was evident (Figure 2A).
Anti-NMO Ab was requested for the patient, which provided a negative result.
Methylprednisolone 500 mg intravenously was started for the patient on March 14, 2019; however, after taking one dose, it was stopped due to the findings of the spiral chest CT scan; it corresponded to a lung infection with COVID- 19 virus. Then, the treatment with IVIG 30 gr/d was initiated for 5 days. On the second day of treatment with IVIG, the strength of the lower limbs increased to 4+/5 MRC; however, with continued treatment, it decreased again in the following days and reached 1/5 MRC.
In the second cervical MRI obtained on March 18, 2019, the previous hyperintense lesion was smaller and shrunken (Figure 2b).
On March 20, 2019, considering the temporary improvement on the second day of treatment with IVIG and the probability of the simultaneous effect of methylprednisolone and IVIG on the improvement of the symptoms, two measures for the patient was taken, as follows:
1. Because about 26 days had passed since the onset symptoms of the disease, an infection specialist was consulted for starting methylprednisolone prescription. The request was not agreed upon due to the findings of the second spiral chest CT scan performed on March 22, 2019 (Figure 1b).
2. It was decided to examine the patient’s CSF and nasopharyngeal swab specimens in terms of 2019-Novel Coronavirus (2019-nCoV) real-time reverse transcriptase-polymerase chain reaction (RT-PCR) on March 21, 2019. The relevant CSF results were suspicious and the nasopharyngeal swab results were negative.
Then, on March 23, 2019, plasmapheresis 1/5 L/d started for 5 days; however, there was no improvement in the patient’s condition and the strength of the lower limbs remained at 1/5 MRC. Eventually, on March 23, the patient was discharged with 1/5 MRC strength of the lower limbs.
Discussion
We presented a case of COVID-19 with myelitis during the pandemic of COVID-19. Moreover, the patient had a fever and gastrointestinal symptoms before presenting neurologic signs.
It is not certain that COVID-19-associated myelitis is an infectious process due to the direct attack of the virus or an immunologic reaction to the virus (para- or post-infection). This differentiation is essential for treatment selection.
There is a theory that COVID-19 can enter the brain microvasculature from the general circulation and the spike protein of the virus could interact with ACE2 expressed in the capillary endothelium and damage to the endothelium could result in viral access to the CNS. This theory is the result of prior research on SARS-CoV [10]. ACE2 receptors are also expressed on the surface of spinal cord cells [11, 12]. Accordingly, it is comprehensible that COVID-19 also involves the spinal cord.
The spinal symptoms appeared after two weeks of the patient’s systemic symptoms; thus, it seems that the myelitis, in this case, is post-infectious, mainly because of normal CSF analysis without pleocytosis or lymphocytosis. This myelitis type occurs with numerous other viruses and bacteria by the immunologic reaction, not a straight attack of the virus to the spinal cord [9, 13, 14].
Additionally, anti-inflammatory approaches may be effective in treating it. However, due to the inadequate evidence, we did not initiate corticosteroid therapy for the patient (considering that the patient’s temporary recovery appeared to be due to the single dose of methylprednisolone or synergistic effects of IVIG). We also had no evidence of the priority of plasmapheresis over IVIG or vice versa to decide the most appropriate first-line treatment.
Furthermore, the patient with myelitis who was reported by Zhao et al. received all antiviral agents, including ganciclovir, lopinavir/ritonavir, moxifloxacin, dexamethasone, human immunoglobulin, and mecobalamin [9]. Although it was reported as post-infectious myelitis.
In this patient, in addition to examining other causes of myelitis, according to the risk factors of atherosclerosis (history of diabetes mellitus, hyperlipidemia, & arterial hypertension), spinal ischemia was also considered. Ischemia mainly affects the anterior spinal cord, especially the thoracic cord, due to the circulatory system of the spinal cord; however, the spinal involvement of this patient did not follow an ischemic pattern.
In this patient, the diagnosis of myelitis due to COVID-19 virus infection or post-infection was made based on history, peripheral ground-glass opacity on spiral chest CT scan, and suspicious CSF PCR for COVID-19 virus. Considering that the myelitis may be post-infectious rather infectious, determining the antibodies against the COVID-19 virus in CSF is more obligatory than the RNA of the virus by PCR assay. Besides, the PCR sensitivity in CSF may be similar to the pharyngeal swab PCR in different stages of the disease mentioned in Xiao study [15]. However, there was no evidence about the sensitivity of COVID-19 virus-PCR assay in CSF. In this patient, at the time of disease, we have no access to antibody assay by ELISA; however, the electrophoresis of CSF demonstrated a high level of gamma globulin as 8.4 mg/dL, i.e. 14.7% (normal range:3.0%-12.0%) of total protein (total protein: 57.4 mg/dL).
In the first case of myelitis reported by Kang Zhao, the test of COVID-19 RNA nasopharyngeal swab was positive. Furthermore, the chest spiral CT demonstrated patchy ground-glass shadow in the anterior segment of the upper lobe of the right lung and patchy high-density shadow in the upper lobe of the left lung . In this case, the serological testing of CSF and spinal cord MRI the were not performed because of special and emergency situation of the pandemia. [9].
A biomarker of the COVID-19 in the CSF or serum of the patients with neurological symptoms is essential and ideal to diagnose cases of COVID-19 with CNS involvement. However, such methods were not available that time. [16].
The patient’s PCR sample of the nasopharyngeal swab was negative after nearly 1 moth post the patient’s fever and systemic symptoms demonstration. According to Xiao, the positive rate of RT-PCR assay was highest at days 0-7 (97.9%), followed by 68.8%, 36.3%, 30.0%, and 26.3% at days 8-14, 15-21, 22-28, and >28, respectively. The median period for the last RT-PCR assay was 24 days (IQR, 20-28; range, 9-48). In Xiao study, male patients had a slightly longer time interval (17 days) of positive SARS-CoV-2 RT-PCR assay than female patients (15 days). Also a risk factor for prolonged virus replication was old age. Older patients (≥65 years) had a significantly longer time interval (18 days) of positive SARS-CoV-2 RT-PCR test results than patients aged <65 years (14 days) [15]. Unfortunately, in this patient, nasopharyngeal swab specimens were not initially taken and the diagnosis of COVID-19 was considered based on the symptoms and the spiral chest CT scan data.
The patient’s spiral chest CT scan at the time of admission was indicative of lung involvement with bilateral and peripheral ground-glass opacities [17]. Although the most apparent abnormalities on chest CT were observable for 10 days, they disappeared 14 days after the onset of symptoms that might last for a longer period [18].
Conclusion
The CNS, including the spinal cord, could be involved by SARS-COV2 directly or through immune reactions. There is no consensus about its treatment. Numerous questions should be answered in future studies. Should the corticosteroids be prescribed to these patients in the post-infectious phase? Does plasma exchange have superiority to IVIG for the treatment of SARS-COV2-associated myelitis?
Should the other therapies for modulating the immune system, like tocilizumab be considered in this situation? Considering the direct involvement of spinal parenchyma, can other compounds suggested for treating of COVID-19, such as remdesivir or ganciclovir, lopinavir/ritonavir, be tried? Notably, we can mention the case reported by Zhao et al., who demonstrated nearly full recovery in his lower limb strength [9].
Furthermore, for the confirmation of SARS-COV2-associated myelitis, the CSF immunologic assay might be more sensitive than the PCR assay of SARS-COV2 RNA.
Eventually, it seems that the definite diagnosis of the type of involvement (direct invasion of virus or immunologic reaction to the virus) of the spine in association with SARS-COV2 should be made by biopsy or autopsy for determining the most effective treatment. As the ACE2 receptors (the target receptor of SARS CoV-2) expresses on endothelium of vessels , it is interesting whether SARS-CoV-2 could be isolated from the endothelium of vessels adjacent to the necrotic areas of the CNS of COVID-19 patients at autopsy [6].
Ethical Considerations
Compliance with ethical guidelines
There was no ethical considerations to be considered in this research.
Funding
This research received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
Conceptualization, design: Alia Saberi, Hamidreza Hatamian; Drafting the manuscript: Alia Saberi; Data collection: Mozaffar Hosseini-Nejad; Data analysis, data interpreting: Babak Bakhshayesh Eghbali; Drafting, revising, and final approval and agreed to be accountable for all aspects of the work: Amirreza Ghayeghran.
Conflict of interest
The authors declared no conflicts of interest.
References
1.Fernández-Garza LE, Marfil A. Neurological aspects that should not be forgotten during the COVID-19 pandemic. Inter Am J Med Health (IAJMH). 2020; 17:3. [DOI:10.31005/iajmh.v3i0.89]
2.Li Z, Huang Y, Guo X. The brain, another potential target organ, needs early protection from SARS-CoV-2 neuroinvasion. Sci China Life Sci. 2020; 63(5):771-3. [DOI:10.1007/s11427-020-1690-y] [PMID] [PMCID]
3.Conde Cardona G, Pájaro LD, Quintero Marzola ID, Ramos Villegas Y, Moscote Salazar L. Neurotropism of SARS-CoV 2: Mechanisms and manifestations. J Neurol Sci. 2020; 412:116824. [DOI: 10.1016/j.jns.2020.116824]
4.Kabbani N, Olds JL. Does COVID19 infect the brain? If so, smokers might be at a higher risk. Mol Pharmacol. 2020; 97(5):351-3. [DOI:10.1124/molpharm.120.000014] [PMID]
5.Li Y, Bai WZ, Hashikawa T. Response to commentary on: ”The neuroinvasive potential of SARS‐CoV‐2 may play a role in the respiratory failure of COVID‐19 patients”. J Med Virol. 2020. [DOI:10.1002/jmv.25824]
6.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020; 201187. [DOI:10.1148/radiol.2020201187] [PMID]
7.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. J Med Virol. 2020 Feb 27. [DOI:10.1002/jmv.25824]
8.Machado C, Gutierrez JV. Brainstem dysfunction in SARS-COV2 infection can be a potential cause of respiratory distress. [DOI:10.20944/preprints202004.0330.v1]
9.Zhao K, Huang J, Dai D, Feng Y, Liu L, Nie Sh. Acute myelitis after SARS-CoV-2 infection: A case report. medRxiv. 2020. [DOI:10.1101/2020.03.16.20035105]
10.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: Tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020; 11(7):995-8. [DOI:10.1021/acschemneuro.0c00122] [PMID] [PMCID]
11.Nemoto W, Yamagata R, Nakagawasai O, Nakagawa K, Hung WY, Fujita M, et al. Effect of spinal angiotensin-converting enzyme 2 activation on the formalin-induced nociceptive response in mice. Eur J Pharmacol. 2020; 872:172950. [DOI:10.1016/j.ejphar.2020.172950] [PMID]
12.Ogata Y, Nemoto W, Yamagata R, Nakagawasai O, Shimoyama S, Furukawa T, et al. Anti-hypersensitive effect of angiotensin (1-7) on streptozotocin-induced diabetic neuropathic pain in mice. Eur J Pain. 2019; 23(4):739-49. [DOI:10.1002/ejp.1341] [PMID]
13.Williams T, Thorpe J. Post-infective transverse myelitis following Streptococcus pneumoniae meningitis with radiological features of acute disseminated encephalomyelitis: A case report. J Med Case Rep. 2012; 6(1):313. [DOI:10.1186/1752-1947-6-313] [PMID] [PMCID]
14.Jacob A, Weinshenker BG. An approach to the diagnosis of acute transverse myelitis. Semin Neurol. 2008; 28(1):105-20. [DOI:10.1055/s-2007-1019132] [PMID]
15.Xiao AT, Tong YX, Gao C, Zhu L, Zhang YJ, Zhang S. Dynamic profile of RT-PCR findings from 301 COVID-19 patients in Wuhan, China: A descriptive study. J Clin Virol. 2020; 127:104346. [DOI:10.1016/j.jcv.2020.104346] [PMID] [PMCID]
16.Baig AM. Neurological manifestations in COVID‐19 caused by SARS‐CoV‐2. CNS Neurosci & Ther. 2020; 26(5):499-501. [DOI:10.1111/cns.13372] [PMID] [PMCID]
17.Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, et al. Chest CT findings in Coronavirus Disease-19 (COVID-19): Relationship to duration of infection. Radiology. 2020; 200463. [DOI:10.1148/radiol.2020200463] [PMID]
18.Liu J, Yu H, Zhang S. The indispensable role of chest CT in the detection of coronavirus disease 2019 (COVID-19). Eur J Nucl Med Mol Imaging. 2020; 3:1. [DOI:10.1007/s00259-020-04795-x]
Received: 2020/05/23 | Accepted: 2020/05/23 | Published: 2020/05/23
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



