Thu, Apr 25, 2024
Volume 5, Issue 4 (Autumn 2019)
Caspian J Neurol Sci 2019, 5(4): 151-160 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Saberi A, Hatamian H, Ghayeghran A, Mola Hosseini F, Noroozi Guilandehi S, Rezaei S et al . Comparing the Quality of Life in Patients With Multiple Sclerosis Consuming Fingolimod and Cinnovex. Caspian J Neurol Sci 2019; 5 (4) :151-160
URL: http://cjns.gums.ac.ir/article-1-291-en.html
URL: http://cjns.gums.ac.ir/article-1-291-en.html
Alia Saberi * 

 1, Hamidreza Hatamian2
1, Hamidreza Hatamian2 
 , Amirreza Ghayeghran2
, Amirreza Ghayeghran2 
 , Fatemeh Mola Hosseini2
, Fatemeh Mola Hosseini2 
 , Sama Noroozi Guilandehi3
, Sama Noroozi Guilandehi3 
 , Sajjad Rezaei4
, Sajjad Rezaei4 

 , Fatemeh Shafaei5
, Fatemeh Shafaei5 



 1, Hamidreza Hatamian2
1, Hamidreza Hatamian2 
 , Amirreza Ghayeghran2
, Amirreza Ghayeghran2 
 , Fatemeh Mola Hosseini2
, Fatemeh Mola Hosseini2 
 , Sama Noroozi Guilandehi3
, Sama Noroozi Guilandehi3 
 , Sajjad Rezaei4
, Sajjad Rezaei4 

 , Fatemeh Shafaei5
, Fatemeh Shafaei5 

1- Neuroscience Research Center, Department of Neurology, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran
2- Department of Neurology, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran
3- School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
4- Department of Psychology, University of Guilan, Rasht, Iran
5- Neuroscience Research Center, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran
2- Department of Neurology, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran
3- School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
4- Department of Psychology, University of Guilan, Rasht, Iran
5- Neuroscience Research Center, Poursina Hospital, Guilan University of Medical Sciences, Rasht, Iran
Full-Text [PDF 1468 kb]
(1166 Downloads)
| Abstract (HTML) (3296 Views)
According to Table 1, the average age of Cinnovex consumers (39.5 y) was significantly higher than the average age of Fingolimod consumers (35.1 y) (t=2.56, P=0.012). There was no significant difference between the two groups regarding the duration of the disease (P>0.05). Finally, the mean age of onset of disease and depression scores were not significantly different between the two groups (P>0.05). Accordingly, the Mann-Whitney U test was used to determine and compare the mean number of attacks in the past 6 months between the two groups. The mean number of attacks in the last 6 months among patients consuming Fingolimod (67.57) was significantly higher than the mean score among patients consuming Cinnovex (33.47) (U=1083, P=0.033).
Table 2 presents the descriptive statistics of quality of life and its dimensions in the two study groups. The Table 2 also reports the results of the Kolmogorov-Smirnov test to verify the normal distribution of variables in the groups. According to the Table 2, the Z statistic of the Kolmogorov-Smirnov test is not significant for all variables. Therefore, the distribution of these variables is normal.
One-Way analysis of covariance was used to investigate the differences in the quality of life in the two groups of Fingolimod and Cinnovex consumers. The results of the homogeneity of the slope regression test in the two groups showed that the regression of the slope was equal in both groups (F=1.02, P=0.31). The results of Levene’s test for homogeneity of the dependent variable showed that the quality of life variance was equal in both groups (F=1.53, P=0.10).
Table 3 reports the results of the univariate analysis of covariance for investigating the difference between the two groups in the quality of life variable. Before performing this analysis and also multivariate covariance to identify confounding variables, the correlation analysis between demographic variables and HAQUAMS total score was performed, and a significant relationship was found between HAQUAMS total score and demographic variables of age (r=0.42). Attacks have occurred in the last 6 months (rho=0.042). Therefore, these variables were considered as confounders in statistical inference analysis.
Results of ANCOVA, after controlling for confounding variables of the “age of disease onset” and “number of attacks in the past six months”, showed that Fingolimod users obtained a lower total score on the quality of life questionnaire (Table 3). MANCOVA was used to investigate the difference in the quality of life between the two study groups. This analysis sought to determine the differences between the components of “fatigue/cognitive function”, “motor problems/lower limbs”, “motor problems/upper limbs”, “social functioning and relationships”, and “mood”.
Before performing this analysis, the Levene’s test results for homogeneity of the dependent variables showed that the components of “fatigue/cognitive function”, (F1,53=7.50, P≤0.008), “motor problems/upper limbs”, (F1,53=18.58, P≤0.0001), and “mood” (F1,53=4.61, P≤0.036) were heterogeneous. But the variances of “motor problems/lower limbs” (F1.53=0.65, P≤0.42) and “social functioning/relationships” (F1.53=0.78, P≤0.38) were homogenous. The results of Box’s M test for checking the equality of the covariance matrix of dependent variables in the study groups showed that the covariance matrix of the dependent variables was not equal between the two groups (Box’s M=42.69, F=2.55, P≤0.001). However, we used MANCOVA for data analysis, because this model in sample sizes larger than 30 per group is resistant to violations of the homogeneity of variance-covariance matrices assumption [22].
Multivariate analysis of covariance on the subscales of the quality of life showed that after controlling confounding variables of the “age of disease onset” and “the number of attacks in the last 6 months”, considering Bonferroni correction only the variable of “mood” was significant (F=6.931, P=0.011, η=0.12). That is to say, consumers of Fingolimod have a better overall mood than Cinnovex users (Table 4). The effect size for the “mood” variable indicates that the difference between the two groups is 0.12. That is, 12% of the variance can be explained regarding group membership. There was no significant difference in the other subscales.
Discussion
In the treatment of MS, the inflammatory immune component of the disease is the primary targeted, without acting directly on the central nervous system [23]. MS medications include Fingolimod, which is the first drug to control the disease, and Cinnovex, a beta-interferon. In this study, we aimed to investigate and compare the effect of these two drugs on the patients’ quality of life.
Because of the lack of access to MS patients and their reluctance to cooperate, the number of patients under study was less than the estimated sample size. One hundred and six patients were enrolled in this study, including 52 patients taking Fingolimod and 54 taking Cinnovex. There were no significant differences between the two groups in the demographic variables of the age of disease onset, duration of disease, gender, marital status, income status, type of drug used, comorbidities, and severity of the disability.
The study groups had significant differences in the level of education and age. HAQUAMS and BDI were used to assess patients’ quality of life and depression index. The mean age of the patients was 37 years, and 70.8% of our study population were women. In the HAQUAMS, the highest and the lowest mean subscales belonged to “fatigue/cognitive function” and “motor problems/upper limb”, respectively. There were no significant differences between the two groups in the subscales of quality of life.
Cognitive problems, especially memory loss, were reported in one-third of patients in the early phase of the disease; this deficiency was also associated with poor quality of life [24]. Another effective thing in lowering the quality of life is physical capacity. In other studies, fatigue and depression were also identified as two major factors affecting the quality of life [24, 25]. Gold et al. used the HAQUAMS to assess the life of MS patients. The results of this study indicated that cognitive problems might be associated with a lower quality of life [26]. The results of our studies showed a positive correlation between depression score and quality of life score so that the higher depression was associated with higher scoring in HAQUAMS, but there was no significant difference between the two groups in this correlation coefficient.
Depression is a serious mental disorder in patients with MS. The precise cause of depression in patients is unknown, but a combination of psychosocial, neurological, and comorbid factors may be involved [27]. It may be due to the direct effect of the inflammation, and the loss of the myelin sheath or the chronic and unpredictable psychological effects of MS. Depression has the highest prevalence in patients with MS [28] and about 50-60% of MS patients suffer from depression, which has a profound negative impact on patients’ quality of life. Social dysfunction is associated with suicidal ideation and worsening disease conditions in these patients [29, 30].
Depression is thus primarily associated with a decline in the quality of life. One of the most important reasons for their association is the fact that people with depressive moods cannot enjoy life as much as other people, so their quality of life will not be as high as the average population of society. On the other hand, depression can lead to sexual problems, loss of energy of the patient, and judging the things and happenings around, and all are items associated with the quality of life [31-33]. The two groups did not differ significantly in the majority of demographic factors, except for the level of education, which was significant. There was no significant difference between the two groups in terms of the severity of the disability. Numerous studies have reported “fatigue” as the most common annoying symptom of MS [34]. Physical disabilities also have a significant impact on the quality of life of patients with MS [35, 36].
In Pfaffenberger et al. study, the association of various factors such as age, sex, disability level, disease duration, and ability to walk with the quality of life-related to patient health was investigated [37]. The study found that female gender, older age, higher disability, and inability to walk were associated with lower quality of life of the patients. Also, increasing the duration of the disease and its progressive course significantly reduced the quality of life of the patients [37].
Statistical analysis after eliminating the effect of confounding variables showed us that Fingolimod users had a better quality of life, considering the total score. Besides, consumers of Fingolimod generally had a better mood than those who took Cinnovex. In Montalban et al. study, Fingolimod significantly improved the quality of life for patients and also reduced depression, which is one of the most important causes of quality of life [38]. There are, however, other studies suggesting that Fingolimod does not have a significant effect on fatigue, which is another symptom affecting the quality of life [39].
Abolfazli et al. found no change in the quality of life during the one year of treatment. Cinnovex is a newer drug than most others and studies on it have not been widely conducted; however, in a study on 1050 patients with RRMS, the results showed that during one year, Cinnovex prevented the progression of the disability, recurrence, and lesion burden in patients [16, 40]. In another study, which examined the clinical efficacy of interferon β1a, it reduced relapse and progressive disability in patients with MS. Similar results were reported in the PRISMS study. Other studies have suggested that beta-interferons harm patients’ quality of life after 2 years [40-42]. In general, Cinnovex seems to have no comparative advantage over Fingolimod, especially in cases of mood swings; we can use Fingolimod in the clinic due to its positive effect on the mood.
One limitation of the present study is the low sample size due to some shortcomings related to time and administrative limitations; also, some patients refused or failed to be cooperative. This drop in sample size could reduce the power of the test to reject the null hypothesis.
Conclusion
Users of Fingolimod had a better quality of life than the other group. Consumers of this drug also had a better mood than the other group. According to some studies, however, the impact of Cinnovex is unclear. Interferon may also improve the course of the disability. Given this difference in the quality of life and improved mood and ease of administration of Fingolimod over the injectable route and the side effects of Cinnovex as an immunological drug, it is reasonable to use Fingolimod in clinical practice.
To improve the data analysis, enough samples of patients with different types of MS must be recruited. Their demographic information and necessary tests must be recorded and taken accurately. Also, during treatment, these tests are repeated to evaluate the effect of the medication on patients.
Ethical Considerations
Compliance with ethical guidelines
The Ethics Committee of Guilan University of Medical Sciences approved the study protocol (No. 1018). In this study, no intervention in medical treatment was performed. All data were classified as confidential and would be published as the study population.
Funding
This article is based on the MD. thesis of Fateme Mola Hosseini on Neurology Specialty (registered thesis number: IR.GUMS.REC.1396.70) funded by Guilan University of Medical Sciences.
Authors' contributions
Drafting the original paper: Sajjad Rezaei and Fateme mola Hosseini; Writing, reviewing, and editing the paper: Sama Noroozi Guilandehi and Alia Saberi; Collecting resources: Sajjad Rezaei and Fatemeh Mola Hosseini, Supervising the research: Hamidreza Hatamian and Alia Saberi; and Data collecting: Fatemeh Mola Hosseini.
Conflict of interest
The authors declared no conflict of interest.
References
Veiga C, Campelo I, Crisóstomo R, Fraga J, Poitier S, Saraiva M. DGI-010 Analysis of the use of fingolimod in patients with Multiple Sclerosis in a University Hospital. Eur J Hosp Pharm. 2013; 20(1): 98-9. [DOI:10.1136/ejhpharm-2013-000276.276]
Gold SM, Heesen C, Schulz H, Guder U, Mönch A, Gbadamosi J, et al. Disease specific quality of life instruments in multiple sclerosis: Validation of the Hamburg Quality of Life Questionnaire in Multiple Sclerosis (HAQUAMS). Mult Scler. 2001; 7(2):119-30. [DOI:10.1191/135245801678227649] [PMID]
Fletcher SG, Castro-Borrero W, Remington G, Treadaway K, Lemack GE, Frohman EM. Sexual dysfunction in patients with multiple sclerosis: A multidisciplinary approach to evaluation and management. Nat Clin Pract Urol. 2009; 6(2):96-107. [DOI:10.1038/ncpuro1298] [PMID]
Ruggeri M, D’Ausilio A, Lo Muto R, Cottone S, Ghezzi A, Mecozzi A, et al. Budget impact analysis of Fingolimod in relapsing remitting Multiple Sclerosis. Value Health. 2014; 17(7):A393. [DOI:10.1016/j.jval.2014.08.872] [PMID]
Nikfar S, Kebriaeezadeh A, Dinarvand R, Abdollahi M, Sahraian MA, Henry D, et al. Cost-effectiveness of different interferon beta products for relapsing-remitting and secondary progressive multiple sclerosis: Decision analysis based on long-term clinical data and switchable treatments. Daru. 2013; 21(1):50. [DOI:10.1186/2008-2231-21-50] [PMID] [PMCID]
Cutter GR, Baier ML, Rudick RA, Cookfair DL, Fischer JS, Petkau J, et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain. 1999; 122(5):871-82. [DOI:10.1093/brain/122.5.871] [PMID]
Niino M. Painful symptoms and quality of life in multiple sclerosis. Neurology Asia. 2008; 13(2):185-7.
Frohman EM, Racke MK, Raine CS. Multiple sclerosis-the plaque and its pathogenesis. N Engl J Med. 2006; 354(9):942-55. [DOI:10.1056/NEJMra052130] [PMID]
Tolpin HG, Bentkover JD. Economic cost of illness: Decision-making applications and practical considerations. Adv Health Econ Health Serv Res. 1983; 4:165-98.
Gajofatto A, Turatti M, Monaco S, Benedetti MD. Clinical efficacy, safety, and tolerability of Fingolimod for the treatment of relapsing-remitting multiple sclerosis. Drug Healthc Patient Saf. 2015; 7:157-67. [DOI:10.2147/DHPS.S69640] [PMID] [PMCID]
Chun J, Hartung HP. Mechanism of action of oral Fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol. 2010; 33(2):91-101. [DOI:10.1097/WNF.0b013e3181cbf825] [PMID] [PMCID]
Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, et al. A placebo-controlled trial of oral Fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010; 362(5):387-401. [DOI:10.1056/NEJMoa0909494] [PMID]
Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, et al. Oral Fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010; 362(5):402-15. [DOI:10.1056/NEJMoa0907839] [PMID]
Calabresi PA, Radue EW, Goodin D, Jeffery D, Rammohan KW, Reder AT, et al. Safety and efficacy of Fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014; 13(6):545-56. [DOI:10.1016/S1474-4422(14)70049-3]
Najafi B, Ghaderi H, Jafari M, Najafi S, Ahmad Kiadaliri A. Cost effectiveness analysis of Avonex and Cinnovex in Relapsing-remitting MS. Glob J Health Sci. 2014; 7(2):139-47. [DOI:10.5539/gjhs.v7n2p139] [PMID] [PMCID]
Etemadifar M, Mazdeh M, Torabi HR, et al. A report of multiple sclerosis patients treated by Cinnovex™ in Iran. Tehran Univ Med J. 2010; 68(1):30-6.
Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011; 69(2):292-302. [DOI:10.1002/ana.22366] [PMID] [PMCID]
Faul F, Erdfelder E, Lang AG, Buchner A. G×Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007; 39(2):175-91.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An Expanded Disability Status Scale (EDSS). Neurology. 1983; 33(11):1444-52. [DOI:10.1212/WNL.33.11.1444] [PMID]
Rajabi Gh. [Psychometric Properties of Beck Depression Inventory Short Form Items (BDI-13) (Persian)]. J Iran Psychol. 2005; 1(4):291-8.
Fathi-Ashtiani A. [Psychological tests: Personality and mental health (Persian)], 1st ed. Tehran: Besat Pub.; 2010.
Allen PJ, Bennett K. SPSS for the health and behavioural sciences. Boston: Thomson Learning; 2007.
Salvetti M, Giovannoni G, Aloisi F. Epstein-Barr virus and multiple sclerosis. Curr Opin Neurol. 2009; 22(3):201-6. [DOI:10.1097/WCO.0b013e32832b4c8d] [PMID]
Pittion-Vouyovitch S, Debouverie M, Guillemin F, et al. Fatigue in multiple sclerosis is related to disability, depression and quality of life. J Neurol Sci 2006; 243(1-2):39-45. [DOI:10.1016/j.jns.2005.11.025] [PMID]
Benedict RH, Wahlig E, Bakshi R, Fishman I, Munschauer F, Zivadinov R, et al. Predicting quality of life in multiple sclerosis: Accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. J Neurol Sci. 2005; 231(1-2):29-34. [DOI:10.1016/j.jns.2004.12.009] [PMID]
Gold SM, Schulz H, Monch A, Schulz KH, Heesen C. Cognitive impairment in multiple sclerosis does not affect reliability and validity of self-report health measures. Mult Scler. 2003; 9(4):404-10. [DOI:10.1191/1352458503ms927oa] [PMID]
Chwastiak LA, Ehde DM. Psychiatric issues in multiple sclerosis. Psychiatr Clin North Am. 2007. 30(4):803-17. [DOI:10.1016/j.psc.2007.07.003] [PMID] [PMCID]
Liu XJ, Ye HX, Li WP, Dai R, Chen D, Jin M. Relationship between psychosocial factors and onset of multiple sclerosis. Eur Neurol. 2009; 62(3):130-6. [DOI:10.1159/000226428] [PMID] [PMCID]
Seyed Ahmadi Nejad FS, Golmakani N, Asghari Pour N, Gandomi F, Malaki Moghadam H, Norozi E. [Effect of Progressive Muscle Relaxation on depression, anxiety, and stress of primigravid women (Persian)]. Evid Based Care J. 2015; 5(1):67-76.
Kirchner T, Lara S. Stress and depression symptoms in patients with multiple sclerosis: The mediating role of the loss 0of social functioning. Acta Neurol Scand. 2011; 123(6):407-13. [DOI:10.1111/j.1600-0404.2010.01422.x] [PMID]
Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: A new measure. Psychopharmacol Bull. 1993; 29(2):321-6. [DOI:10.1037/t49981-000]
Siegert RJ, Abernethy DA. Depression in multiple sclerosis: A review. J Neurol Neurosurg Psychiatry. 2005; 76(4):469-75. [DOI:10.1136/jnnp.2004.054635] [PMID] [PMCID]
Janardhan V, Bakshi R. Quality of life in patients with multiple sclerosis: the impact of fatigue and depression. J Neurol Sci. 2002; 205(1):51-8. [DOI:10.1016/S0022-510X(02)00312-X]
Masoodi R, Mohammadi E, Nabavi S, Ahmadi F. [The effect of Orem based self-care program on physical quality of life in multiple sclerosis patients (Persian)]. J Shahrekord Uuniv Med Sci. 2008; 10(2):21-9.
Rousseaux M, Pérennou D. Comfort care in severely disabled multiple sclerosis patients. J Neurol Sci. 2004; 222(1):39-48. [DOI:10.1016/j.jns.2004.04.002] [PMID]
Haresabadi M, Karimi Monaghi H, Froghipor M, Mazlom SR. [Quality of life in patients with Multiple Sclerosis referring to Ghaem Hospital, Mashhad in 2009 (Persian)]. J North Khorasan Univ Med Sci. 2011; 2(4):7-12. [DOI:10.29252/jnkums.2.4.7]
Pfaffenberger N, Pfeiffer KP, Deibl M, Höfer S, Günther V, Ulmer H. Association of factors influencing health-related quality of life in MS. Acta Neurol Scand. 2006; 114(2):102-8. [DOI:10.1111/j.1600-0404.2006.00659.x] [PMID]
Montalban X, Comi G, O’Connor P, Gold S, de Vera A, Eckert B, et al. Oral Fingolimod (FTY720) in relapsing multiple sclerosis: Impact on health-related quality of life in a phase II study. Mult Scler. 2011; 17(11):1341-50. [DOI:10.1177/1352458511411061] [PMID]
Masingue M, Debs R, Maillart E, Delvaux V, Lubetzki C, Vidal JS. Fatigue evaluation in Fingolimod treated patients: An observational study. Mult Scler Relat Disord. 2017; 14:8-11. [DOI:10.1016/j.msard.2017.03.006] [PMID]
Ebers GC. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet. 1998; 352(9139):1498-504. [DOI:10.1016/S0140-6736(98)03334-0]
Vermersch P, de Seze J, Delisse B, Lemaire S, Stojkovic T, et al. Quality of life in multiple sclerosis: Influence of interferon-beta1 a (Avonex) treatment. Mult Scler. 2002; 8(5):377-81. [DOI:10.1191/1352458502ms826oa] [PMID]
Rice GP, Oger J, Duquette P, Bélanger M, Laplante S, Grenier JF. Treatment with interferon beta-1b improves quality of life in multiple sclerosis. Can J Neurol Sci. 1999; 26(4):276-82. [DOI:10.1017/S031716710000038X] [PMID]
Full-Text: (968 Views)
Introduction
Multiple Sclerosis (MS) is characterized by chronic demyelination, inflammatory, and degenerative disease of the central nervous system [1, 2] characterized by recurrent episodes of neurological impairment and disability that are sometimes reversible, sometimes irreversible, and sometimes both of them [2]. It is the most common progressive neurologic disability in young adults [3]. The exact etiology of the disease is still unclear, but the activation of immune mechanisms against myelin antigens is involved in the disease [4].
Currently, more than 1.3 million people worldwide suffer from MS [3]. Iran is also one of the countries where the prevalence of MS is moderate [5]; also, MS is 1.4 to 3.1 times more frequent in women than men [2]. The disease has an unpredictable course and affects the productive years of young people. Therapies can only slow or control the clinical signs, but MS has psychological effects on the patients [6]: Restrict their participation in health-related activities, limit their independent lives, and ultimately hurt their quality of life [7]. In the treatment of MS, the inflammatory immune component of the disease is targeted without affecting the central nervous system [8].
In recent decades, two preventive medications have been introduced and approved: 1. Subcutaneous glatiramer acetate injection, and 2. Three types of interferon-β: intramuscular interferon β1a (low dose), subcutaneous β1a (high-dose), and subcutaneous β1b [9].
After almost two decades of using only injectable drugs for MS, Fingolimod (in the form of capsules) was introduced as the first disease-modifying drug that had a promising effect in the treatment of MS [10]. Fingolimod exerts its therapeutic effects by modifying the Sphingosine-1-Phosphate (S1P) receptor [11]. Initial data indicate that Fingolimod significantly reduces the recurrence rate, suppresses inflammatory activity based on brain MRI, and slows brain atrophy in MS patients compared to placebo and intramuscular interferon [12-14].
One of the interferons is beta-Cinnovex, a form of beta-interferon 1a. A recombinant protein containing 166 amino acids and weighing 22.5 kDa is produced by Chinese hamster ovary cells [15]. Cinnovex is an alternative medicine that has been produced in Iran in recent years and has shown satisfactory results. Studies have shown that the drug inhibits the progression of disability in patients with MS and controls its relapse and recurrence [16]. Due to the injectable use of all previous MS drugs, including Cinnovex and the difficulty of repeated injections and the short-term and long-term side effects resulting from the injections, patients tend to take oral Fingolimod.
This study aimed to evaluate and compare the quality of life of the patients of Guilan MS Society who use Fingolimod with those who use Cinnovex, one of the most commonly used injectable drugs. Notably, no similar study has been conducted in Iran yet, and even the quality of life of patients with consuming Fingolimod alone has not been evaluated. Monitoring and measuring the quality of life of MS patients is of great importance regarding the outcome of their drug treatment.
Restrictions that may occur to patients in the course of their illness can have a devastating impact on their independent lives and degrade their quality of life and accelerate their disability. The purpose of this study was to compare the quality of life in patients with MS using Fingolimod and Cinnovex.
Materials and Methods
This analytic-cross-sectional study was performed on patients with Relapsing-Remitting Multiple Sclerosis (RRMS) treated with Fingolimod or Cinnovex referred to neurology clinics of Guilan University of Medical Sciences in 1977. A neurologist confirmed this diagnosis in the participants based on the 2011 McDonald criteria [17].
Method of sampling and calculation of sample size
We used the G×Power V. 3.1.9.2 to determine the sample size with respect to the F ratio, the main effect of the independent variable (i.e. the type of drug administered), and the inclusion of at least three covariates (i.e. age, sex, duration of disease) [18]. Given the α=0.05, test power=0.95, and effect size=0.07, G×Power calculated the sample size as 122 (the optimal sample size in each drug consuming group as 61).
The inclusion criteria were using those drugs for at least 6 months and being able to answer the study questions. The exclusion criteria were having severe psychiatric disorders, including psychosis, based on the DSM-5 diagnostic criteria, and clinical depression (severe) based on the short form of Beck depression inventory (score above 16).
Study tools
The data collection tool consisted of three questionnaires.
Demographic information
It collects data regarding age, gender, marital status (single, widowed, divorced, about to divorced), location (rural, urban), age of disease onset, duration of MS (month), number of attacks in the past 6 months, the Expanded Disability Status Scale (EDSS) score of the patient, comorbidities, economic status, and education level. Clinicians use EDSS to evaluate the central nervous system function of the MS patient, the progression of MS, and the effectiveness of therapeutic interventions.
The scoring of this scale starts from 0=healthy nervous system to 10=death due to MS. This scale increases by half a unit and is scored based on the mean scores of the motor, cognitive, visual, brainstem function, sensory function, cerebellar function, and ability to control urine and stool. Scores of 0-1.5 refer to no disability, 2-2.5 denotes mild disability, 3-5.5 indicates moderate disability, and the score of more than 5.5 is considered severe disability [19].
Short-form Beck Depression Inventory
Beck Depression Inventory (BDI) measures depression symptoms in clinical and research settings [20]. It has 13 self-report items that measure the cognitive, emotional, and physical dimensions of depression. Each item in the inventory contains a four-item scale ranging from 0 to 3, with a maximum score of 39.
According to factor analysis, two factors of negative self-esteem and dissatisfaction were identified in the Iranian samples. The Cronbach alpha for this questionnaire was 0.89, and its correlation coefficient with the long form of the same test (BDI-21) was 0.67. The evidence suggests that the short form of BDI is eligible for use in psychological research and depression screening in the normal Iranian population [21]. In this study, the Cronbach alpha coefficient of this questionnaire was 0.86, and the BDI-13 cut-off point of 16 and higher were used to separate adolescent girls with the depressed mood from other samples [21].
Hamburg Quality of Life Questionnaire in Multiple Sclerosis
Gold et al. developed this tool in 2001. The questionnaire consists of 38 items, of which 28 basic items in 5 subsets reflect essential dimensions of quality of life in MS patients: fatigue/cognitive functioning (4 items); lower limb movements (5 items); upper limb movements (5 items); communication (6 items); and mood (8 items). The items were scored in each subscale on a 5-point Likert-type scale. Lower scores indicate better quality of life. The scores of all items in a subscale are added and then divided into the number of items to obtain the total score for that subscale.
Similarly, the total score of Hamburg Quality of Life Questionnaire in MS (HAQUAMS), which ranges from 1-5, is calculated. Meanwhile, items 34, 35, 36, and 37 have a reverse scoring. Ten items are not included in calculating the total score of HAQUAMS, but provide additional information on sensory functions, including urine, stool, and sexual control, major illness symptoms, recent changes in the patient’s health, vision impairment, and general rating; however, it is not a major component of quality of life and is not included in the overall score [2].
The questionnaire was presented to 10 faculty member neurologists and neuroscience specialists after translating. The CVR obtained from all questions was 0.8 based on the Lawshe CVR, except for one question (question No. 25), in which the obtained CVR reviewed by the specialists was 0.60. Also, the CVI score in terms of simplicity and clarity in all questions was 0.93, indicating that the content validity of the scale was confirmed. The Cronbach alpha coefficient for items involved in scoring in the Persian version was calculated as 0.929.
Study procedure
After enrolling the subjects, the researchers obtained informed written consent from the patients. Before conducting the study, they explained the objectives and study procedure to all patients. Completing the questionnaire for each person took about 15-25 minutes. Two medical students (one female and one male) interviewed the subjects on visiting days at the Neurology Clinic of Guilan University of Medical Sciences from 3 to 8 PM. for 5 hours. The evaluator read and explained all the items and their scoring to the patient and then recorded the subjects’ verbal responses. The researchers stayed in the adjacent neurologist’s room on the visiting days, and patients were referred to the evaluation room after the neurologist’s visit. All patients entered the study with full and informed consent. Names, specifications, and all information of the patients were kept confidential.
Statistical analysis
The demographic and medical characteristics of the patients in the two groups of Fingolimod and Cinnovex were compared by the Pearson Chi-square test for categorical variables and the independent t-test for continuous variables. Mann-Whitney U test was used where comparisons were needed.
To compare the two groups of patients in terms of quality of life and its five dimensions, after adjusting for possible demographic differences, we used One-Way Analysis of Covariance (ANCOVA) and Multivariate Analysis (MANCOVA). Before that, the Pearson and Spearman correlation analyses were used to find confounding variables associated with HAQUAMS scores. The obtained data were analyzed in SPSS V. 22.
Results
After excluding ineligible participants, 106 patients with MS (52 using Fingolimod and 54 using Cinnovex) were enrolled in this study. The mean age of study patients was 37 years (range: 21-64 years, 70.8% female). About 84.9% of the patients were living in urban and 14.2% in rural areas. Also, 54% of patients were housekeeper or unemployed and had no income, or were supported by relief organizations. The income of 10.4% of them was under $100, 6.6% between $100 and $300, and 27.4% over $300 per month. About 50.9% of the patients had a college education, 25.5% high school diploma, 22.6% a senior high school diploma, and 0.9% were illiterate. After evaluating the severity of disability by EDSS, it is found that 45 (42.4%) patients had no disability, 23 (21.69%) mild disability, 26 (24.52%) moderate disability, and 12 (11.32%) had a severe disability.
Based on the Chi-square test, the two groups were not significantly different in terms of gender, marital status, income, the severity of the disability, place of residence, and comorbidities (P>0.05). Nevertheless, the value of this test was significant after comparing the educational level (χ2=12.63, P=0.002). So that consumers of Fingolimod had a higher level of education than Cinnovex consumers.
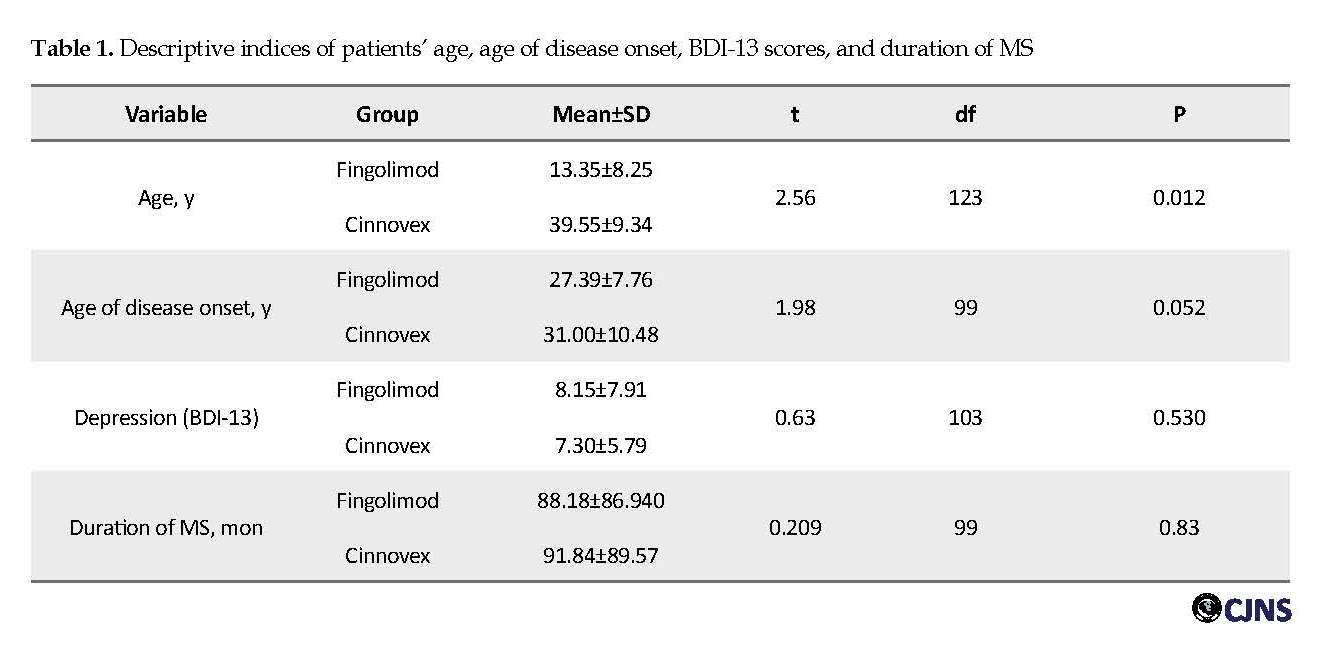
The independent samples t-test was used to investigate the mean age, age of onset, duration of disease in MS patients using Fingolimod, and using Cinnovex (Table 1).
Multiple Sclerosis (MS) is characterized by chronic demyelination, inflammatory, and degenerative disease of the central nervous system [1, 2] characterized by recurrent episodes of neurological impairment and disability that are sometimes reversible, sometimes irreversible, and sometimes both of them [2]. It is the most common progressive neurologic disability in young adults [3]. The exact etiology of the disease is still unclear, but the activation of immune mechanisms against myelin antigens is involved in the disease [4].
Currently, more than 1.3 million people worldwide suffer from MS [3]. Iran is also one of the countries where the prevalence of MS is moderate [5]; also, MS is 1.4 to 3.1 times more frequent in women than men [2]. The disease has an unpredictable course and affects the productive years of young people. Therapies can only slow or control the clinical signs, but MS has psychological effects on the patients [6]: Restrict their participation in health-related activities, limit their independent lives, and ultimately hurt their quality of life [7]. In the treatment of MS, the inflammatory immune component of the disease is targeted without affecting the central nervous system [8].
In recent decades, two preventive medications have been introduced and approved: 1. Subcutaneous glatiramer acetate injection, and 2. Three types of interferon-β: intramuscular interferon β1a (low dose), subcutaneous β1a (high-dose), and subcutaneous β1b [9].
After almost two decades of using only injectable drugs for MS, Fingolimod (in the form of capsules) was introduced as the first disease-modifying drug that had a promising effect in the treatment of MS [10]. Fingolimod exerts its therapeutic effects by modifying the Sphingosine-1-Phosphate (S1P) receptor [11]. Initial data indicate that Fingolimod significantly reduces the recurrence rate, suppresses inflammatory activity based on brain MRI, and slows brain atrophy in MS patients compared to placebo and intramuscular interferon [12-14].
One of the interferons is beta-Cinnovex, a form of beta-interferon 1a. A recombinant protein containing 166 amino acids and weighing 22.5 kDa is produced by Chinese hamster ovary cells [15]. Cinnovex is an alternative medicine that has been produced in Iran in recent years and has shown satisfactory results. Studies have shown that the drug inhibits the progression of disability in patients with MS and controls its relapse and recurrence [16]. Due to the injectable use of all previous MS drugs, including Cinnovex and the difficulty of repeated injections and the short-term and long-term side effects resulting from the injections, patients tend to take oral Fingolimod.
This study aimed to evaluate and compare the quality of life of the patients of Guilan MS Society who use Fingolimod with those who use Cinnovex, one of the most commonly used injectable drugs. Notably, no similar study has been conducted in Iran yet, and even the quality of life of patients with consuming Fingolimod alone has not been evaluated. Monitoring and measuring the quality of life of MS patients is of great importance regarding the outcome of their drug treatment.
Restrictions that may occur to patients in the course of their illness can have a devastating impact on their independent lives and degrade their quality of life and accelerate their disability. The purpose of this study was to compare the quality of life in patients with MS using Fingolimod and Cinnovex.
Materials and Methods
This analytic-cross-sectional study was performed on patients with Relapsing-Remitting Multiple Sclerosis (RRMS) treated with Fingolimod or Cinnovex referred to neurology clinics of Guilan University of Medical Sciences in 1977. A neurologist confirmed this diagnosis in the participants based on the 2011 McDonald criteria [17].
Method of sampling and calculation of sample size
We used the G×Power V. 3.1.9.2 to determine the sample size with respect to the F ratio, the main effect of the independent variable (i.e. the type of drug administered), and the inclusion of at least three covariates (i.e. age, sex, duration of disease) [18]. Given the α=0.05, test power=0.95, and effect size=0.07, G×Power calculated the sample size as 122 (the optimal sample size in each drug consuming group as 61).
The inclusion criteria were using those drugs for at least 6 months and being able to answer the study questions. The exclusion criteria were having severe psychiatric disorders, including psychosis, based on the DSM-5 diagnostic criteria, and clinical depression (severe) based on the short form of Beck depression inventory (score above 16).
Study tools
The data collection tool consisted of three questionnaires.
Demographic information
It collects data regarding age, gender, marital status (single, widowed, divorced, about to divorced), location (rural, urban), age of disease onset, duration of MS (month), number of attacks in the past 6 months, the Expanded Disability Status Scale (EDSS) score of the patient, comorbidities, economic status, and education level. Clinicians use EDSS to evaluate the central nervous system function of the MS patient, the progression of MS, and the effectiveness of therapeutic interventions.
The scoring of this scale starts from 0=healthy nervous system to 10=death due to MS. This scale increases by half a unit and is scored based on the mean scores of the motor, cognitive, visual, brainstem function, sensory function, cerebellar function, and ability to control urine and stool. Scores of 0-1.5 refer to no disability, 2-2.5 denotes mild disability, 3-5.5 indicates moderate disability, and the score of more than 5.5 is considered severe disability [19].
Short-form Beck Depression Inventory
Beck Depression Inventory (BDI) measures depression symptoms in clinical and research settings [20]. It has 13 self-report items that measure the cognitive, emotional, and physical dimensions of depression. Each item in the inventory contains a four-item scale ranging from 0 to 3, with a maximum score of 39.
According to factor analysis, two factors of negative self-esteem and dissatisfaction were identified in the Iranian samples. The Cronbach alpha for this questionnaire was 0.89, and its correlation coefficient with the long form of the same test (BDI-21) was 0.67. The evidence suggests that the short form of BDI is eligible for use in psychological research and depression screening in the normal Iranian population [21]. In this study, the Cronbach alpha coefficient of this questionnaire was 0.86, and the BDI-13 cut-off point of 16 and higher were used to separate adolescent girls with the depressed mood from other samples [21].
Hamburg Quality of Life Questionnaire in Multiple Sclerosis
Gold et al. developed this tool in 2001. The questionnaire consists of 38 items, of which 28 basic items in 5 subsets reflect essential dimensions of quality of life in MS patients: fatigue/cognitive functioning (4 items); lower limb movements (5 items); upper limb movements (5 items); communication (6 items); and mood (8 items). The items were scored in each subscale on a 5-point Likert-type scale. Lower scores indicate better quality of life. The scores of all items in a subscale are added and then divided into the number of items to obtain the total score for that subscale.
Similarly, the total score of Hamburg Quality of Life Questionnaire in MS (HAQUAMS), which ranges from 1-5, is calculated. Meanwhile, items 34, 35, 36, and 37 have a reverse scoring. Ten items are not included in calculating the total score of HAQUAMS, but provide additional information on sensory functions, including urine, stool, and sexual control, major illness symptoms, recent changes in the patient’s health, vision impairment, and general rating; however, it is not a major component of quality of life and is not included in the overall score [2].
The questionnaire was presented to 10 faculty member neurologists and neuroscience specialists after translating. The CVR obtained from all questions was 0.8 based on the Lawshe CVR, except for one question (question No. 25), in which the obtained CVR reviewed by the specialists was 0.60. Also, the CVI score in terms of simplicity and clarity in all questions was 0.93, indicating that the content validity of the scale was confirmed. The Cronbach alpha coefficient for items involved in scoring in the Persian version was calculated as 0.929.
Study procedure
After enrolling the subjects, the researchers obtained informed written consent from the patients. Before conducting the study, they explained the objectives and study procedure to all patients. Completing the questionnaire for each person took about 15-25 minutes. Two medical students (one female and one male) interviewed the subjects on visiting days at the Neurology Clinic of Guilan University of Medical Sciences from 3 to 8 PM. for 5 hours. The evaluator read and explained all the items and their scoring to the patient and then recorded the subjects’ verbal responses. The researchers stayed in the adjacent neurologist’s room on the visiting days, and patients were referred to the evaluation room after the neurologist’s visit. All patients entered the study with full and informed consent. Names, specifications, and all information of the patients were kept confidential.
Statistical analysis
The demographic and medical characteristics of the patients in the two groups of Fingolimod and Cinnovex were compared by the Pearson Chi-square test for categorical variables and the independent t-test for continuous variables. Mann-Whitney U test was used where comparisons were needed.
To compare the two groups of patients in terms of quality of life and its five dimensions, after adjusting for possible demographic differences, we used One-Way Analysis of Covariance (ANCOVA) and Multivariate Analysis (MANCOVA). Before that, the Pearson and Spearman correlation analyses were used to find confounding variables associated with HAQUAMS scores. The obtained data were analyzed in SPSS V. 22.
Results
After excluding ineligible participants, 106 patients with MS (52 using Fingolimod and 54 using Cinnovex) were enrolled in this study. The mean age of study patients was 37 years (range: 21-64 years, 70.8% female). About 84.9% of the patients were living in urban and 14.2% in rural areas. Also, 54% of patients were housekeeper or unemployed and had no income, or were supported by relief organizations. The income of 10.4% of them was under $100, 6.6% between $100 and $300, and 27.4% over $300 per month. About 50.9% of the patients had a college education, 25.5% high school diploma, 22.6% a senior high school diploma, and 0.9% were illiterate. After evaluating the severity of disability by EDSS, it is found that 45 (42.4%) patients had no disability, 23 (21.69%) mild disability, 26 (24.52%) moderate disability, and 12 (11.32%) had a severe disability.
Based on the Chi-square test, the two groups were not significantly different in terms of gender, marital status, income, the severity of the disability, place of residence, and comorbidities (P>0.05). Nevertheless, the value of this test was significant after comparing the educational level (χ2=12.63, P=0.002). So that consumers of Fingolimod had a higher level of education than Cinnovex consumers.
The independent samples t-test was used to investigate the mean age, age of onset, duration of disease in MS patients using Fingolimod, and using Cinnovex (Table 1).
According to Table 1, the average age of Cinnovex consumers (39.5 y) was significantly higher than the average age of Fingolimod consumers (35.1 y) (t=2.56, P=0.012). There was no significant difference between the two groups regarding the duration of the disease (P>0.05). Finally, the mean age of onset of disease and depression scores were not significantly different between the two groups (P>0.05). Accordingly, the Mann-Whitney U test was used to determine and compare the mean number of attacks in the past 6 months between the two groups. The mean number of attacks in the last 6 months among patients consuming Fingolimod (67.57) was significantly higher than the mean score among patients consuming Cinnovex (33.47) (U=1083, P=0.033).
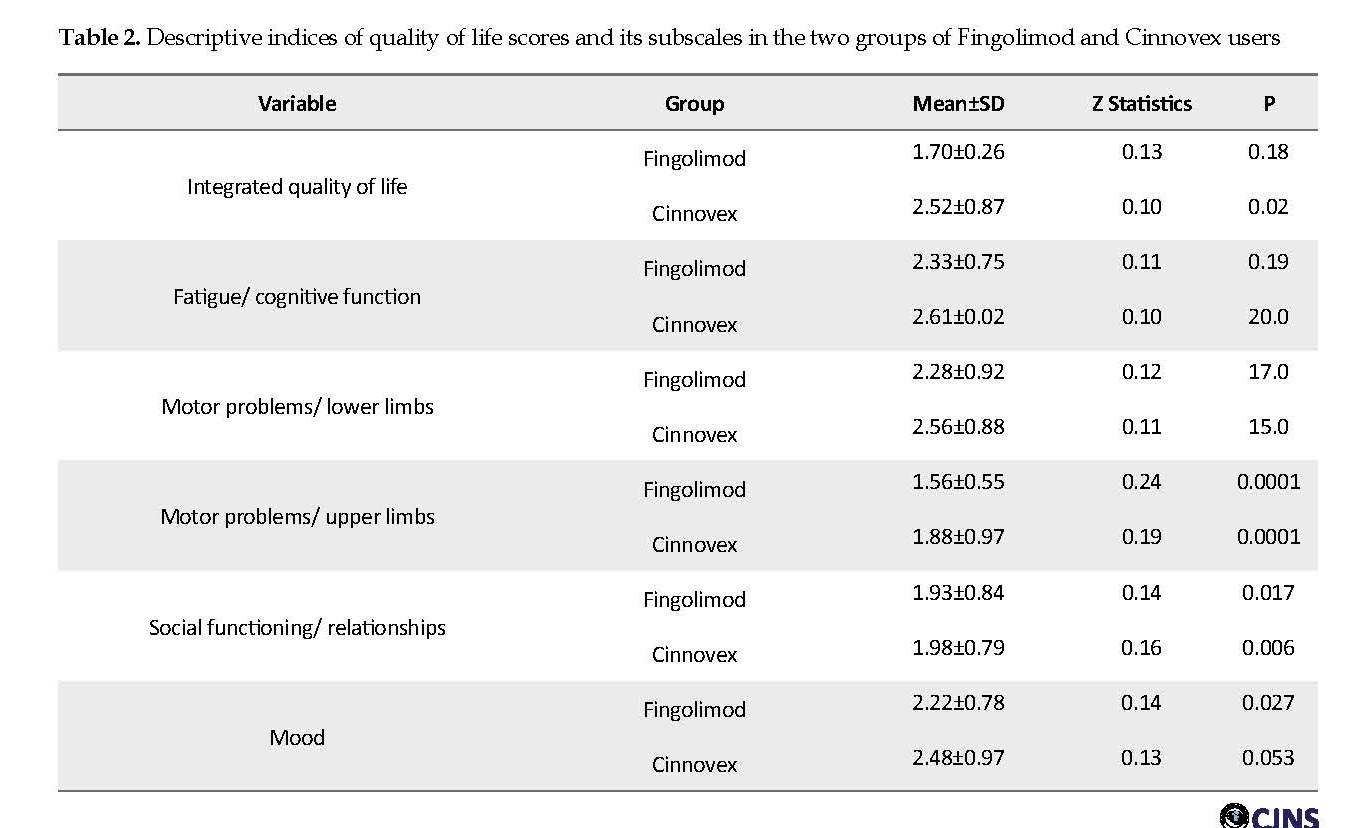
Table 2 presents the descriptive statistics of quality of life and its dimensions in the two study groups. The Table 2 also reports the results of the Kolmogorov-Smirnov test to verify the normal distribution of variables in the groups. According to the Table 2, the Z statistic of the Kolmogorov-Smirnov test is not significant for all variables. Therefore, the distribution of these variables is normal.
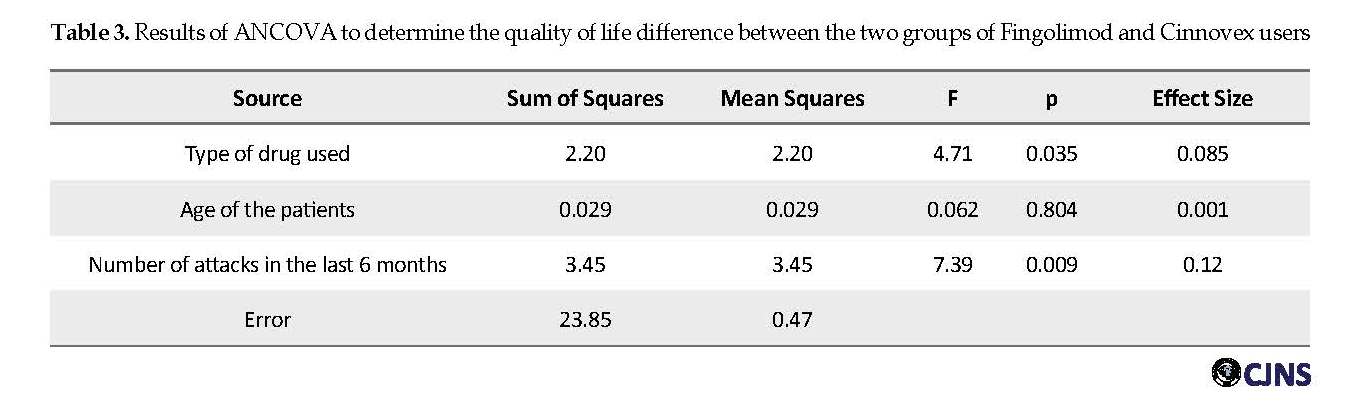
One-Way analysis of covariance was used to investigate the differences in the quality of life in the two groups of Fingolimod and Cinnovex consumers. The results of the homogeneity of the slope regression test in the two groups showed that the regression of the slope was equal in both groups (F=1.02, P=0.31). The results of Levene’s test for homogeneity of the dependent variable showed that the quality of life variance was equal in both groups (F=1.53, P=0.10).
Table 3 reports the results of the univariate analysis of covariance for investigating the difference between the two groups in the quality of life variable. Before performing this analysis and also multivariate covariance to identify confounding variables, the correlation analysis between demographic variables and HAQUAMS total score was performed, and a significant relationship was found between HAQUAMS total score and demographic variables of age (r=0.42). Attacks have occurred in the last 6 months (rho=0.042). Therefore, these variables were considered as confounders in statistical inference analysis.
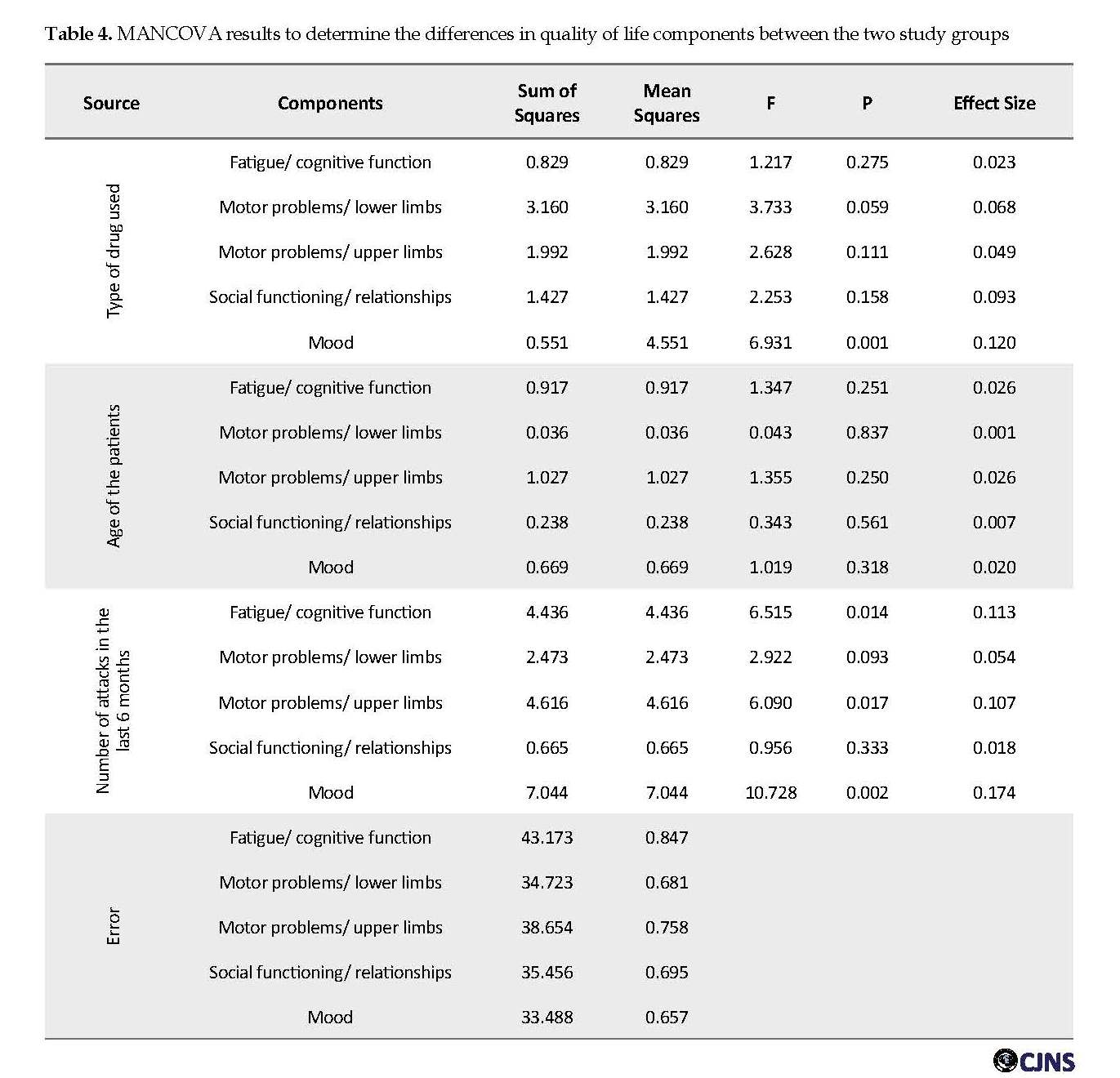
Results of ANCOVA, after controlling for confounding variables of the “age of disease onset” and “number of attacks in the past six months”, showed that Fingolimod users obtained a lower total score on the quality of life questionnaire (Table 3). MANCOVA was used to investigate the difference in the quality of life between the two study groups. This analysis sought to determine the differences between the components of “fatigue/cognitive function”, “motor problems/lower limbs”, “motor problems/upper limbs”, “social functioning and relationships”, and “mood”.
Before performing this analysis, the Levene’s test results for homogeneity of the dependent variables showed that the components of “fatigue/cognitive function”, (F1,53=7.50, P≤0.008), “motor problems/upper limbs”, (F1,53=18.58, P≤0.0001), and “mood” (F1,53=4.61, P≤0.036) were heterogeneous. But the variances of “motor problems/lower limbs” (F1.53=0.65, P≤0.42) and “social functioning/relationships” (F1.53=0.78, P≤0.38) were homogenous. The results of Box’s M test for checking the equality of the covariance matrix of dependent variables in the study groups showed that the covariance matrix of the dependent variables was not equal between the two groups (Box’s M=42.69, F=2.55, P≤0.001). However, we used MANCOVA for data analysis, because this model in sample sizes larger than 30 per group is resistant to violations of the homogeneity of variance-covariance matrices assumption [22].
Multivariate analysis of covariance on the subscales of the quality of life showed that after controlling confounding variables of the “age of disease onset” and “the number of attacks in the last 6 months”, considering Bonferroni correction only the variable of “mood” was significant (F=6.931, P=0.011, η=0.12). That is to say, consumers of Fingolimod have a better overall mood than Cinnovex users (Table 4). The effect size for the “mood” variable indicates that the difference between the two groups is 0.12. That is, 12% of the variance can be explained regarding group membership. There was no significant difference in the other subscales.
Discussion
In the treatment of MS, the inflammatory immune component of the disease is the primary targeted, without acting directly on the central nervous system [23]. MS medications include Fingolimod, which is the first drug to control the disease, and Cinnovex, a beta-interferon. In this study, we aimed to investigate and compare the effect of these two drugs on the patients’ quality of life.
Because of the lack of access to MS patients and their reluctance to cooperate, the number of patients under study was less than the estimated sample size. One hundred and six patients were enrolled in this study, including 52 patients taking Fingolimod and 54 taking Cinnovex. There were no significant differences between the two groups in the demographic variables of the age of disease onset, duration of disease, gender, marital status, income status, type of drug used, comorbidities, and severity of the disability.
The study groups had significant differences in the level of education and age. HAQUAMS and BDI were used to assess patients’ quality of life and depression index. The mean age of the patients was 37 years, and 70.8% of our study population were women. In the HAQUAMS, the highest and the lowest mean subscales belonged to “fatigue/cognitive function” and “motor problems/upper limb”, respectively. There were no significant differences between the two groups in the subscales of quality of life.
Cognitive problems, especially memory loss, were reported in one-third of patients in the early phase of the disease; this deficiency was also associated with poor quality of life [24]. Another effective thing in lowering the quality of life is physical capacity. In other studies, fatigue and depression were also identified as two major factors affecting the quality of life [24, 25]. Gold et al. used the HAQUAMS to assess the life of MS patients. The results of this study indicated that cognitive problems might be associated with a lower quality of life [26]. The results of our studies showed a positive correlation between depression score and quality of life score so that the higher depression was associated with higher scoring in HAQUAMS, but there was no significant difference between the two groups in this correlation coefficient.
Depression is a serious mental disorder in patients with MS. The precise cause of depression in patients is unknown, but a combination of psychosocial, neurological, and comorbid factors may be involved [27]. It may be due to the direct effect of the inflammation, and the loss of the myelin sheath or the chronic and unpredictable psychological effects of MS. Depression has the highest prevalence in patients with MS [28] and about 50-60% of MS patients suffer from depression, which has a profound negative impact on patients’ quality of life. Social dysfunction is associated with suicidal ideation and worsening disease conditions in these patients [29, 30].
Depression is thus primarily associated with a decline in the quality of life. One of the most important reasons for their association is the fact that people with depressive moods cannot enjoy life as much as other people, so their quality of life will not be as high as the average population of society. On the other hand, depression can lead to sexual problems, loss of energy of the patient, and judging the things and happenings around, and all are items associated with the quality of life [31-33]. The two groups did not differ significantly in the majority of demographic factors, except for the level of education, which was significant. There was no significant difference between the two groups in terms of the severity of the disability. Numerous studies have reported “fatigue” as the most common annoying symptom of MS [34]. Physical disabilities also have a significant impact on the quality of life of patients with MS [35, 36].
In Pfaffenberger et al. study, the association of various factors such as age, sex, disability level, disease duration, and ability to walk with the quality of life-related to patient health was investigated [37]. The study found that female gender, older age, higher disability, and inability to walk were associated with lower quality of life of the patients. Also, increasing the duration of the disease and its progressive course significantly reduced the quality of life of the patients [37].
Statistical analysis after eliminating the effect of confounding variables showed us that Fingolimod users had a better quality of life, considering the total score. Besides, consumers of Fingolimod generally had a better mood than those who took Cinnovex. In Montalban et al. study, Fingolimod significantly improved the quality of life for patients and also reduced depression, which is one of the most important causes of quality of life [38]. There are, however, other studies suggesting that Fingolimod does not have a significant effect on fatigue, which is another symptom affecting the quality of life [39].
Abolfazli et al. found no change in the quality of life during the one year of treatment. Cinnovex is a newer drug than most others and studies on it have not been widely conducted; however, in a study on 1050 patients with RRMS, the results showed that during one year, Cinnovex prevented the progression of the disability, recurrence, and lesion burden in patients [16, 40]. In another study, which examined the clinical efficacy of interferon β1a, it reduced relapse and progressive disability in patients with MS. Similar results were reported in the PRISMS study. Other studies have suggested that beta-interferons harm patients’ quality of life after 2 years [40-42]. In general, Cinnovex seems to have no comparative advantage over Fingolimod, especially in cases of mood swings; we can use Fingolimod in the clinic due to its positive effect on the mood.
One limitation of the present study is the low sample size due to some shortcomings related to time and administrative limitations; also, some patients refused or failed to be cooperative. This drop in sample size could reduce the power of the test to reject the null hypothesis.
Conclusion
Users of Fingolimod had a better quality of life than the other group. Consumers of this drug also had a better mood than the other group. According to some studies, however, the impact of Cinnovex is unclear. Interferon may also improve the course of the disability. Given this difference in the quality of life and improved mood and ease of administration of Fingolimod over the injectable route and the side effects of Cinnovex as an immunological drug, it is reasonable to use Fingolimod in clinical practice.
To improve the data analysis, enough samples of patients with different types of MS must be recruited. Their demographic information and necessary tests must be recorded and taken accurately. Also, during treatment, these tests are repeated to evaluate the effect of the medication on patients.
Ethical Considerations
Compliance with ethical guidelines
The Ethics Committee of Guilan University of Medical Sciences approved the study protocol (No. 1018). In this study, no intervention in medical treatment was performed. All data were classified as confidential and would be published as the study population.
Funding
This article is based on the MD. thesis of Fateme Mola Hosseini on Neurology Specialty (registered thesis number: IR.GUMS.REC.1396.70) funded by Guilan University of Medical Sciences.
Authors' contributions
Drafting the original paper: Sajjad Rezaei and Fateme mola Hosseini; Writing, reviewing, and editing the paper: Sama Noroozi Guilandehi and Alia Saberi; Collecting resources: Sajjad Rezaei and Fatemeh Mola Hosseini, Supervising the research: Hamidreza Hatamian and Alia Saberi; and Data collecting: Fatemeh Mola Hosseini.
Conflict of interest
The authors declared no conflict of interest.
References
Veiga C, Campelo I, Crisóstomo R, Fraga J, Poitier S, Saraiva M. DGI-010 Analysis of the use of fingolimod in patients with Multiple Sclerosis in a University Hospital. Eur J Hosp Pharm. 2013; 20(1): 98-9. [DOI:10.1136/ejhpharm-2013-000276.276]
Gold SM, Heesen C, Schulz H, Guder U, Mönch A, Gbadamosi J, et al. Disease specific quality of life instruments in multiple sclerosis: Validation of the Hamburg Quality of Life Questionnaire in Multiple Sclerosis (HAQUAMS). Mult Scler. 2001; 7(2):119-30. [DOI:10.1191/135245801678227649] [PMID]
Fletcher SG, Castro-Borrero W, Remington G, Treadaway K, Lemack GE, Frohman EM. Sexual dysfunction in patients with multiple sclerosis: A multidisciplinary approach to evaluation and management. Nat Clin Pract Urol. 2009; 6(2):96-107. [DOI:10.1038/ncpuro1298] [PMID]
Ruggeri M, D’Ausilio A, Lo Muto R, Cottone S, Ghezzi A, Mecozzi A, et al. Budget impact analysis of Fingolimod in relapsing remitting Multiple Sclerosis. Value Health. 2014; 17(7):A393. [DOI:10.1016/j.jval.2014.08.872] [PMID]
Nikfar S, Kebriaeezadeh A, Dinarvand R, Abdollahi M, Sahraian MA, Henry D, et al. Cost-effectiveness of different interferon beta products for relapsing-remitting and secondary progressive multiple sclerosis: Decision analysis based on long-term clinical data and switchable treatments. Daru. 2013; 21(1):50. [DOI:10.1186/2008-2231-21-50] [PMID] [PMCID]
Cutter GR, Baier ML, Rudick RA, Cookfair DL, Fischer JS, Petkau J, et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain. 1999; 122(5):871-82. [DOI:10.1093/brain/122.5.871] [PMID]
Niino M. Painful symptoms and quality of life in multiple sclerosis. Neurology Asia. 2008; 13(2):185-7.
Frohman EM, Racke MK, Raine CS. Multiple sclerosis-the plaque and its pathogenesis. N Engl J Med. 2006; 354(9):942-55. [DOI:10.1056/NEJMra052130] [PMID]
Tolpin HG, Bentkover JD. Economic cost of illness: Decision-making applications and practical considerations. Adv Health Econ Health Serv Res. 1983; 4:165-98.
Gajofatto A, Turatti M, Monaco S, Benedetti MD. Clinical efficacy, safety, and tolerability of Fingolimod for the treatment of relapsing-remitting multiple sclerosis. Drug Healthc Patient Saf. 2015; 7:157-67. [DOI:10.2147/DHPS.S69640] [PMID] [PMCID]
Chun J, Hartung HP. Mechanism of action of oral Fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol. 2010; 33(2):91-101. [DOI:10.1097/WNF.0b013e3181cbf825] [PMID] [PMCID]
Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, et al. A placebo-controlled trial of oral Fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010; 362(5):387-401. [DOI:10.1056/NEJMoa0909494] [PMID]
Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, et al. Oral Fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010; 362(5):402-15. [DOI:10.1056/NEJMoa0907839] [PMID]
Calabresi PA, Radue EW, Goodin D, Jeffery D, Rammohan KW, Reder AT, et al. Safety and efficacy of Fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014; 13(6):545-56. [DOI:10.1016/S1474-4422(14)70049-3]
Najafi B, Ghaderi H, Jafari M, Najafi S, Ahmad Kiadaliri A. Cost effectiveness analysis of Avonex and Cinnovex in Relapsing-remitting MS. Glob J Health Sci. 2014; 7(2):139-47. [DOI:10.5539/gjhs.v7n2p139] [PMID] [PMCID]
Etemadifar M, Mazdeh M, Torabi HR, et al. A report of multiple sclerosis patients treated by Cinnovex™ in Iran. Tehran Univ Med J. 2010; 68(1):30-6.
Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011; 69(2):292-302. [DOI:10.1002/ana.22366] [PMID] [PMCID]
Faul F, Erdfelder E, Lang AG, Buchner A. G×Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007; 39(2):175-91.
Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An Expanded Disability Status Scale (EDSS). Neurology. 1983; 33(11):1444-52. [DOI:10.1212/WNL.33.11.1444] [PMID]
Rajabi Gh. [Psychometric Properties of Beck Depression Inventory Short Form Items (BDI-13) (Persian)]. J Iran Psychol. 2005; 1(4):291-8.
Fathi-Ashtiani A. [Psychological tests: Personality and mental health (Persian)], 1st ed. Tehran: Besat Pub.; 2010.
Allen PJ, Bennett K. SPSS for the health and behavioural sciences. Boston: Thomson Learning; 2007.
Salvetti M, Giovannoni G, Aloisi F. Epstein-Barr virus and multiple sclerosis. Curr Opin Neurol. 2009; 22(3):201-6. [DOI:10.1097/WCO.0b013e32832b4c8d] [PMID]
Pittion-Vouyovitch S, Debouverie M, Guillemin F, et al. Fatigue in multiple sclerosis is related to disability, depression and quality of life. J Neurol Sci 2006; 243(1-2):39-45. [DOI:10.1016/j.jns.2005.11.025] [PMID]
Benedict RH, Wahlig E, Bakshi R, Fishman I, Munschauer F, Zivadinov R, et al. Predicting quality of life in multiple sclerosis: Accounting for physical disability, fatigue, cognition, mood disorder, personality, and behavior change. J Neurol Sci. 2005; 231(1-2):29-34. [DOI:10.1016/j.jns.2004.12.009] [PMID]
Gold SM, Schulz H, Monch A, Schulz KH, Heesen C. Cognitive impairment in multiple sclerosis does not affect reliability and validity of self-report health measures. Mult Scler. 2003; 9(4):404-10. [DOI:10.1191/1352458503ms927oa] [PMID]
Chwastiak LA, Ehde DM. Psychiatric issues in multiple sclerosis. Psychiatr Clin North Am. 2007. 30(4):803-17. [DOI:10.1016/j.psc.2007.07.003] [PMID] [PMCID]
Liu XJ, Ye HX, Li WP, Dai R, Chen D, Jin M. Relationship between psychosocial factors and onset of multiple sclerosis. Eur Neurol. 2009; 62(3):130-6. [DOI:10.1159/000226428] [PMID] [PMCID]
Seyed Ahmadi Nejad FS, Golmakani N, Asghari Pour N, Gandomi F, Malaki Moghadam H, Norozi E. [Effect of Progressive Muscle Relaxation on depression, anxiety, and stress of primigravid women (Persian)]. Evid Based Care J. 2015; 5(1):67-76.
Kirchner T, Lara S. Stress and depression symptoms in patients with multiple sclerosis: The mediating role of the loss 0of social functioning. Acta Neurol Scand. 2011; 123(6):407-13. [DOI:10.1111/j.1600-0404.2010.01422.x] [PMID]
Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: A new measure. Psychopharmacol Bull. 1993; 29(2):321-6. [DOI:10.1037/t49981-000]
Siegert RJ, Abernethy DA. Depression in multiple sclerosis: A review. J Neurol Neurosurg Psychiatry. 2005; 76(4):469-75. [DOI:10.1136/jnnp.2004.054635] [PMID] [PMCID]
Janardhan V, Bakshi R. Quality of life in patients with multiple sclerosis: the impact of fatigue and depression. J Neurol Sci. 2002; 205(1):51-8. [DOI:10.1016/S0022-510X(02)00312-X]
Masoodi R, Mohammadi E, Nabavi S, Ahmadi F. [The effect of Orem based self-care program on physical quality of life in multiple sclerosis patients (Persian)]. J Shahrekord Uuniv Med Sci. 2008; 10(2):21-9.
Rousseaux M, Pérennou D. Comfort care in severely disabled multiple sclerosis patients. J Neurol Sci. 2004; 222(1):39-48. [DOI:10.1016/j.jns.2004.04.002] [PMID]
Haresabadi M, Karimi Monaghi H, Froghipor M, Mazlom SR. [Quality of life in patients with Multiple Sclerosis referring to Ghaem Hospital, Mashhad in 2009 (Persian)]. J North Khorasan Univ Med Sci. 2011; 2(4):7-12. [DOI:10.29252/jnkums.2.4.7]
Pfaffenberger N, Pfeiffer KP, Deibl M, Höfer S, Günther V, Ulmer H. Association of factors influencing health-related quality of life in MS. Acta Neurol Scand. 2006; 114(2):102-8. [DOI:10.1111/j.1600-0404.2006.00659.x] [PMID]
Montalban X, Comi G, O’Connor P, Gold S, de Vera A, Eckert B, et al. Oral Fingolimod (FTY720) in relapsing multiple sclerosis: Impact on health-related quality of life in a phase II study. Mult Scler. 2011; 17(11):1341-50. [DOI:10.1177/1352458511411061] [PMID]
Masingue M, Debs R, Maillart E, Delvaux V, Lubetzki C, Vidal JS. Fatigue evaluation in Fingolimod treated patients: An observational study. Mult Scler Relat Disord. 2017; 14:8-11. [DOI:10.1016/j.msard.2017.03.006] [PMID]
Ebers GC. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet. 1998; 352(9139):1498-504. [DOI:10.1016/S0140-6736(98)03334-0]
Vermersch P, de Seze J, Delisse B, Lemaire S, Stojkovic T, et al. Quality of life in multiple sclerosis: Influence of interferon-beta1 a (Avonex) treatment. Mult Scler. 2002; 8(5):377-81. [DOI:10.1191/1352458502ms826oa] [PMID]
Rice GP, Oger J, Duquette P, Bélanger M, Laplante S, Grenier JF. Treatment with interferon beta-1b improves quality of life in multiple sclerosis. Can J Neurol Sci. 1999; 26(4):276-82. [DOI:10.1017/S031716710000038X] [PMID]
Type of Study: Research |
Subject:
Special
Received: 2019/04/10 | Accepted: 2019/08/15 | Published: 2019/10/1
Received: 2019/04/10 | Accepted: 2019/08/15 | Published: 2019/10/1
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |