Tue, Apr 23, 2024
Volume 5, Issue 4 (Autumn 2019)
Caspian J Neurol Sci 2019, 5(4): 190-198 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Pourkhani T, Daneshmandi H, Norasteh A A, Bakhshayesh Eghbali B, Sedaghati P. The Effect of Cognitive and Motor Dual-task Training on Improvement of Balance and Some Spatiotemporal Gait Parameters in People With Idiopathic Parkinson's Disease. Caspian J Neurol Sci 2019; 5 (4) :190-198
URL: http://cjns.gums.ac.ir/article-1-281-en.html
URL: http://cjns.gums.ac.ir/article-1-281-en.html
Tahereh Pourkhani * 

 1, Hassan Daneshmandi2
1, Hassan Daneshmandi2 

 , Ali Asghar Norasteh2
, Ali Asghar Norasteh2 

 , Babak Bakhshayesh Eghbali3
, Babak Bakhshayesh Eghbali3 

 , Parisa Sedaghati2
, Parisa Sedaghati2 




 1, Hassan Daneshmandi2
1, Hassan Daneshmandi2 

 , Ali Asghar Norasteh2
, Ali Asghar Norasteh2 

 , Babak Bakhshayesh Eghbali3
, Babak Bakhshayesh Eghbali3 

 , Parisa Sedaghati2
, Parisa Sedaghati2 


1- Department of Corrective Exercises and Sport Injuries, Faculty of Physical Education and Sport Science, University of Guilan, Rasht, Iran , zpourkhani@gmail.com
2- Department of Corrective Exercises and Sport Injuries, Faculty of Physical Education and Sport Science, University of Guilan, Rasht, Iran
3- Department of Neurology, Neuroscience Research Center, Poursina Hospital, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
2- Department of Corrective Exercises and Sport Injuries, Faculty of Physical Education and Sport Science, University of Guilan, Rasht, Iran
3- Department of Neurology, Neuroscience Research Center, Poursina Hospital, School of Medicine, Guilan University of Medical Sciences, Rasht, Iran
Full-Text [PDF 1423 kb]
(907 Downloads)
| Abstract (HTML) (2141 Views)
Regarding gait analysis, improvement in gait velocity can result from increased cadence, increased stride length, or both. The results of our study revealed that stride length and cadence improved with both single and dual-task training. As we expected, significant effects were also observed for other spatiotemporal gait parameters that are strictly related to gait velocity, stride length, and cadence.
For example, we observed a significant decrease in stride time and stance time percentage. In another study, Strouwen et al. compared the efficacy of integrated dual-task training and consecutive dual-task training on gait parameters and risk of fall in patients with PD. They concluded that consecutive and integrated dual-task training could lead to similar improvements in dual-task gait velocity without increasing fall risk. Therefore, these findings support the application of dual-task training in clinical practice [11]. A dual task directs the performer’s attention toward an external source of attention, while performing a primary task. As stated in constrained action hypothesis, this attentional change might allow motor systems to function automatically, resulting in more effective performance [27].
In contrast to our hypothesis, no significant difference was found between study groups. In the current research, even the control group was trained. It seems that the lack of an actual control group without any intervention can be a reason for achieving these results. As reported by studies of motor learning, the effect of DT training will transfer not only to the DT performance but also to the single-Task performance [14]. That could be another reason for lack of any significant difference between (Single-Task control group) STCG and DT training groups in our study. Although we used GPower software for calculating sample size, choosing a small sample size is another limitation of this research, which can affect the study results.
Conclusion
Single task and motor/cognitive dual-task training were equally effective in improvement of balance and some spatiotemporal gait parameters in people with PD. The positive effects retained for one month that was indicative of motor learning capacity in PD. Contrary to current belief, DT training is not hazardous. Thus, DT training should be included in rehabilitation programs by physiotherapists in their clinical practice.
Ethical Considerations
Compliance with ethical guidelines
The study protocol was approved by the Ethics Committee of Guilan University of Medical Sciences (No.IR.GUMS.REC.1396.381), registered at the Iranian Registry of Clinical Trials (IRCT20180106038239N1) and conducted after obtaining the necessary permissions. All the study procedures were in compliance with the ethical guidelines of the Declaration of Helsinki 2013.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
Drafting the paper: Tahereh Pourkhani, Hassan Daneshmandi, Ali Asghar Norasteh; Data collection: Tahereh Pourkhani; Writing the review and editing: Tahereh Pourkhani, Hassan Daneshmandi, Ali Asghar Norasteh; Resources: Tahereh Pourkhani, Hassan Daneshmandi, Ali Asghar Norasteh, Babak Bakhshayesh Eghbali, Parisa Sedaghati; Supervision: Hassan Daneshmandi, Ali Asghar Norasteh, Babak Bakhshayesh Eghbali, Parisa Sedaghati.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors express sincere gratitude toward the personnel of The University of Guilan and Guilan University of Medical Science for their dedicated support.
References
Schapira AHV, Olanow CW. Neuroprotection in Parkinson’s disease: Mysteries, myths, and misconceptions. JAMA. 2004; 291(3):358-64. [DOI:10.1001/jama.291.3.358] [PMID]
Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007; 68(5):384-6. [DOI:10.1212/01.wnl.0000247740.47667.03] [PMID]
Santos AAL, Campos C, Bento T, Lattari E, Egidio Nardi A, Barbosa F, et al. Effects of dual-task interventions on gait performance of patients with Parkinson’s disease: A systematic review. Med Express. 2016; 3(4):1-10. [DOI:10.5935/MedicalExpress.2016.04.01]
Peterson DS, King LA, Cohen RG, Horak FB. Cognitive contributions to freezing of gait in Parkinson disease: Implications for physical rehabilitation. Phys Ther. 2016. 96(5):659-70. [DOI:10.2522/ptj.20140603] [PMID] [PMCID]
Brauer SG, Woollacott MH, Lamont R, Brauer SG, Woollacott MH, Lamont R, et al. Single and dual task gait training in people with Parkinson’s disease: A protocol for a randomised controlled trial. BMC Neurol. 2011; 11:90. [DOI:10.1186/1471-2377-11-90] [PMID] [PMCID]
Ashburn A, Stack E, Ballinger C, Fazakarley L, Fitton C. The circumstances of falls among people with Parkinson’s disease and the use of falls diaries to facilitate reporting. Disabil Rehabil. 2008; 30(16):1205-12. [DOI:10.1080/09638280701828930] [PMID]
Lord S, Galna B, Rochester L. Moving forward on gait measurement: toward a more refined approach. Mov Disord. 2013; 28(11):1534-43. [DOI:10.1002/mds.25545] [PMID]
Heinzel S, Maechtel M, Hasmann SE, Hobert MA, Heger T, Berg D, et al. Motor dual-tasking deficits predict falls in Parkinson’s Disease: A prospective study. Parkinsonism Relat Disord. 2016; 26:73-7. [DOI:10.1016/j.parkreldis.2016.03.007] [PMID]
Spildooren J, Vercruysse S, Desloovere K, Vandenberghe W, Kerckhofs E, Nieuwboer A. Freezing of gait in Parkinson’s Disease: The impact of dual-tasking and turning. Mov Disord. 2010; 25(15):2563-70. [DOI:10.1002/mds.23327] [PMID]
Fuller RL, Van Winkle EP, Anderson KE. Dual task performance in Parkinson’s Disease: A sensitive predictor of impairment and disability. Parkinsonism Relat Disord. 2013; 19(3):325-8. [DOI:10.1016/j.parkreldis.2012.11.011] [PMID]
Strouwen C, Molenaar EALM, Munks L, Keus SHJ, Zijlmans JCM, Vandenberghe W, et al. Training Dual Tasks Together or apart in Parkinson’s Disease: Results From the DUALITY trial. Mov Disord. 2017; 32(8):1201-10. [DOI:10.1002/mds.27014] [PMID]
Wu T, Chan P, Hallett M. Effective connectivity of neural networks in automatic movements in Parkinson’s Disease. Neuroimage. 2010; 49(3):2581-7. [DOI:10.1016/j.neuroimage.2009.10.051] [PMID] [PMCID]
De Freitas TB, Leite PHW, Dona F, Pompeu JE, Swarowsky A, Torriani-Pasin C. The effects of dual task gait and balance training in Parkinson’s Disease: A systematic review. Physiother Theory Pract. 2018; 3:1-9. [DOI:10.1080/09593985.2018.1551455] [PMID]
Kelly VE, Eusterbrock AJ, Shumway-Cook A. The effects of instructionson dual-task walking and cognitive task performance in people with Parkinson’s Disease. Parkinsons Dis. 2012; 2012:1-9. [DOI:10.1155/2012/671261] [PMID] [PMCID]
Cunningham JB, McCrum-Gardner E, Gardner E. Power, effect and sample size using GPower: Practical issues for researchers and members of research ethics committees. Evid Based Midwifery. 2007; 5(4):132-7.
van den Heuvel MRC, Kwakkel G, Beek PJ, Berendse HW, Daffertshofer A, van Wegen EE. Effects of augmented visual feedback during balance training in Parkinson’s Disease: A pilot randomized clinical trial. Parkinsonism Relat Disord. 2014; 2014(43):1352-8. [DOI:10.1016/j.parkreldis.2014.09.022] [PMID]
Yang Y, Hao Y, Tian W, Yang Y, Hao YL, Tian WJ, et al. The effectiveness of Tai Chi for patients with Parkinson’s Disease: Study protocol for a randomized controlled trial. Bio Med Central. 2015; 16:111. [DOI:10.1186/s13063-015-0639-8] [PMID] [PMCID]
Nocera JR, Stegemöller EL, Malaty IA, Okun MS, Marsiske M, Hass CJ, et al. Using the Timed Up & Go Test in a clinical setting to predict falling in Parkinson’s Disease. Arch Phys Med Rehabil. 2013; 94(7):1300-5. [DOI:10.1016/j.apmr.2013.02.020] [PMID] [PMCID]
Fonda B, Sarabon N, Li FX. Validity and reliability of different kinematics methods used for bike fitting. J Sports Sci. 2014; 32(10):40-96. [DOI:10.1080/02640414.2013.868919] [PMID]
Sanudo B, Rueda D, del Pozo-Cruz B, Carrasco L. Validation of A video analysis software package for quantifying movement velocity in resistance exercises. J Strength Cond Res. 2016; 30(10):2934-41. [DOI:10.1519/JSC.0000000000000563] [PMID]
Sacheli MA, Murray DK, Vafai N. Habitual exercisers versus sedentary subjects with Parkinson’s Disease: Multimodal PET and fMRI study. Move Disord. 2018; 32(12):1945-50. [DOI:10.1002/mds.27498] [PMID]
Canning GC, Ada L, Woodhouse E. Multiple-task walking training in people with mild to moderate Parkinson’s Disease: A pilot study. Clin Rehabil. 2008; 22(3):226-33. [DOI:10.1177/0269215507082341] [PMID]
Wild LB, de Lima DB, Balardin JB. Freezing of gait in Parkinson’s Disease: The impact of dual-tasking and turning. Mov Disord. 2013; 25(15):2563-70 [DOI:10.1002/mds.23327] [PMID]
Keus SH, Bloem BR, Hendriks EJ. Evidence-based analysis of physical therapy in Parkinson’s Disease with recommendations for practice and research. Move Disord. 2007; 22(4):451-60. [DOI:10.1002/mds.21244] [PMID]
Keus S, Munneke M, Graziano M, Paltamaa J, Pelosin E, Domingos J, et al. European physiotherapy guideline for Parkinson’s Disease. The Netherlands: KNGF/ParkinsonNet; 2014.
Rios Romenets S, Anang J, Fereshtehnejad SM, Pelletier A, Postuma R. Tango for treatment of motor and non-motor manifestations in Parkinson’s Disease: Randomized control study. Complement Ther Med. 2015; 23(2):175-84. [DOI:10.1016/j.ctim.2015.01.015] [PMID]
Ghai S, Ghai I, Effenberg A. Effects of dual tasks and dual-task training on postural stability: A systematic review and meta-analysis. Clin Interv Aging. 2017; 12:557-77. [DOI:10.2147/CIA.S125201] [PMID] [PMCID]
Full-Text: (870 Views)
Introduction
Parkinson disease (PD) is the second most common neurodegenerative disorder after Alzheimer disease that targets basal ganglia [1]. Four million people now live with PD all over the world, and this number will double until 2030 [2]. This illness is characterized by both motor and non-motor symptoms that disrupt activities of daily living [1]. The main motor symptoms include tremor, rigidity, bradykinesia, and postural instability [3]. Patients with PD usually experience cognitive impairments, including deficits in executive function, attention, working memory, and visuospatial domains that can interfere with their mobility [4].
More than half of the patients with PD suffer from gait disturbances depending on their disease severity that causes falling so that between 50% to 68% of people with PD experience at least one fall during a year [5, 6]. Apparently gait impairments like reduced stride length and slower gait velocity are common in patients with PD [7]. Some medications, such as levodopa is the standard treatment for PD, but they become less effective for some symptoms after a long period of usage [5]. Thus, the other kinds of treatments like exercise therapy or physical therapy are needed for these patients.
Dual-Task (DT) performance refers to performing two tasks at the same time. Mobility in daily life frequently requires DT performances, such as talking with carrying a glass of water while walking. Several studies demonstrated some gait disturbances such as increased risk of falling, more freezing of gait, and reduced functional mobility during DT conditions in patients with PD [8-10]. According to the guidelines of physical therapy, DT training is better avoided or used cautiously in PD because its practicality is unclear [11]. Because of loss of automaticity in PD, patients find it challenging to do two tasks at the same time [12]. However, some recent studies showed benefits of DT training in PD patients [13].
Santos et al. in a systematic review, reported that different types of dual-task interventions could improve some gait parameters in patients with PD [3]. As mentioned above, since the people in their activities of daily living face with DT conditions a lot, DT training could be beneficial, especially in patients with balance and gait disorders like PD. Another point is that research on the ability to modify DT performance among people with PD is very limited [14]. Therefore, this study investigates whether cognitive and motor DT training has any effect on balance and some spatiotemporal gait parameters in people with idiopathic PD.
Materials and Methods
Study design
The study was performed with the approval of the University of Guilan and registered in the Iranian Registry of Clinical Trials (No. IRCT20180106038239N1). A single-blind controlled trial was conducted to compare cognitive and motor dual-task training with simple-task trining. The research period included 10 weeks supervised training and one-month follow-up and primary outcomes measured at baseline, after supervised training, and one month later (as follow-up). In this study, the patients were trained and assessed by a physiotherapist in a private clinic of physical therapy. Also, the patients were tested on medications condition, 1-2 hours after taking antiparkinson medications, at the same time of day for pre- and post-intervention and follow up assessments.
Study participants
Considering α=0.05, effect size: 0.5, and analysis power of 0.8, the sample size was estimated as 30 by GPower 3.1 (an excellent freeware program for sample size analysis) [15]. So, thirty patients with mild to moderate PD were recruited for the study. After evaluating the eligibility of the patients, they were informed of the study procedure and signed informed written consent before the study. The patients were assigned to a control group (n=10) and 2 experimental groups (n=20). In experimental groups, there were 10 patients in motor dual-task training group (MDTTG) and 10 patients in cognitive dual-task training group (CDTTG).
Inclusion criteria
Diagnosis of PD by a neurologist, being at stage II-III based on the Hoehn and Yahr scale [16]. Aged between 50 to 75 years. Under stable medication regimen within the previous month and during the period of the research (4 months) [17]. Able to walk 100 m independently without any assistive devices [5].
Exclusion criteria
Having another neurological condition in addition to PD. Suffering from any musculoskeletal or cardiopulmonary conditions that affect the quality of life. Undergoing surgery for PD such as deep brain stimulation. Getting a score of less than 24 in the mini-mental status examination. Having sensory impairment (e.g. blindness, deafness) [5]. Having participated in an organized exercise therapy program in the last 6 months [17].
Outcome measures
In the current study, the outcome measures were timed up and go test (TUG) and some spatiotemporal gait parameters, including stride length (cm), cadence (step/min), stride time (stride/s), swing % (% of gait cycle), and stance % (% of gait cycle). TUG test is a functional test the measures the ability of the patient to rise from a seated position on a chair, walk 3 m, turn, walk back, and sit down. This test requires only a few minutes to accomplish, easy to administer, and requires a few pieces of equipment. In PD, longer TUG test times are associated with decreased mobility. Also, TUG test has a high test-retest reliability and interrater reliability in PD population [18]. For assessing some spatiotemporal gait parameters, HD VideoCam-Kinovea was used.
The motion was simultaneously recorded using two Casio EX-F1 HD VideoCam in the sagittal and frontal plane with the ability to shoot 1920×1080 full HD movies at 60 frames per second. One camera (in the sagittal plane) was placed on a tripod at the height of 50 cm at a distance of 2 m to the center of the pathway to capture a good view of a gait cycle. Another camera was placed in front of the subject at a distance of 3 m [19]. This setting ensured that the calibration area covered the lower limb of the subject (field of view). The outputs (video recordings) from the two HD VideoCam were fed into Kinovea 0.8.25 (an open-source software) to analyze the gait. It seems that HD VideoCam-Kinovea is a reliable motion capture-analysis system (r=0.79). Moreover, it is low cost, portable, and easy to use [20].
Study itervention
In the single-Task training control group (STTCG) and cognitive and motor Dual-Task Training (CDTTG and MDTTG) groups, the patients participated in a 30 session program administered for 45 minutes each session, 3 times per week for 10 weeks. The training was conducted by a physiotherapist in the “on medication” condition (often 1 hour after medication). In the single-Task group, the patients made a selection of exercises presented by Parkinson society of Canada, including wall standing exercise, tandem stance, single-leg stance, standing on toes, squat, march, side bending exercise, trunk rotation exercise, and figure of eight walking [21]. According to overload principle of exercise, training program had a progressive trend.
In the cognitive and motor dual-task groups, the patients did the exercises while performing various additional cognitive or motor tasks. Other cognitive tasks during training included counting backward by 3 s, memory recall, generating category lists (e.g. fruit, sports, names starting with a specific letter) and simple calculation tasks. Additional motor tasks were selected to reflect everyday activities such as doing up buttons, carrying a plate with a glass on top, and transferring coins between pockets or objects like cell phone between hands while training [22]. The patients were instructed to perform additional tasks correctly while doing the exercises.
Statistical analysis
Statistical analysis was performed in SPSS V. 20. The results obtained in the study were considered statistically significant at P≤0.05. For assessing any differences between clinical and demographic variables at the beginning of the study, One-Way analysis of variance (ANOVA) was used. Assumptions of data normality were checked before the analysis by the Shapiro-Wilk test. The data collected from the single-Task group statistically compared with the two dual-task training groups. In this research, for comparison of the effects of single and motor/ cognitive dual-task training on the balance and some spatiotemporal gait parameters, six separate repeated measures ANOVA were conducted. Also, Greenhouse-Geisser correction was used when the obtained results from the Mauchly’s test of sphericity indicated a violation of the sphericity assumption (P≤0.05). Post hoc analyses were carried out using Bonferroni corrections for multiple comparisons.
Results
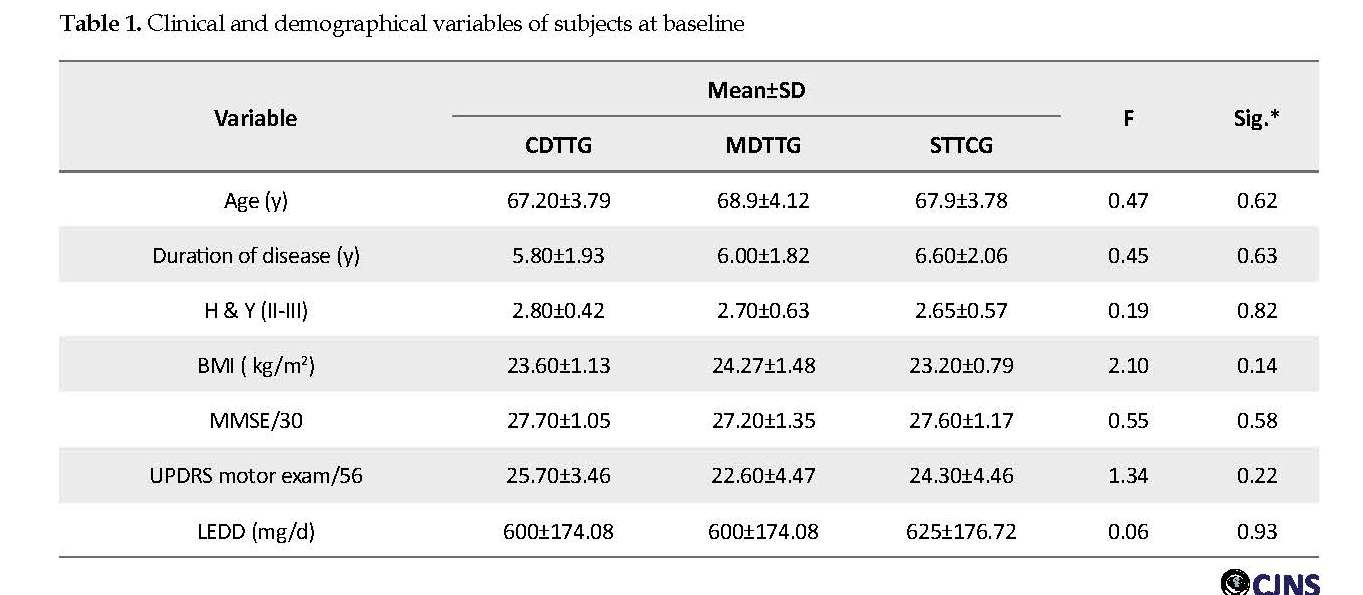
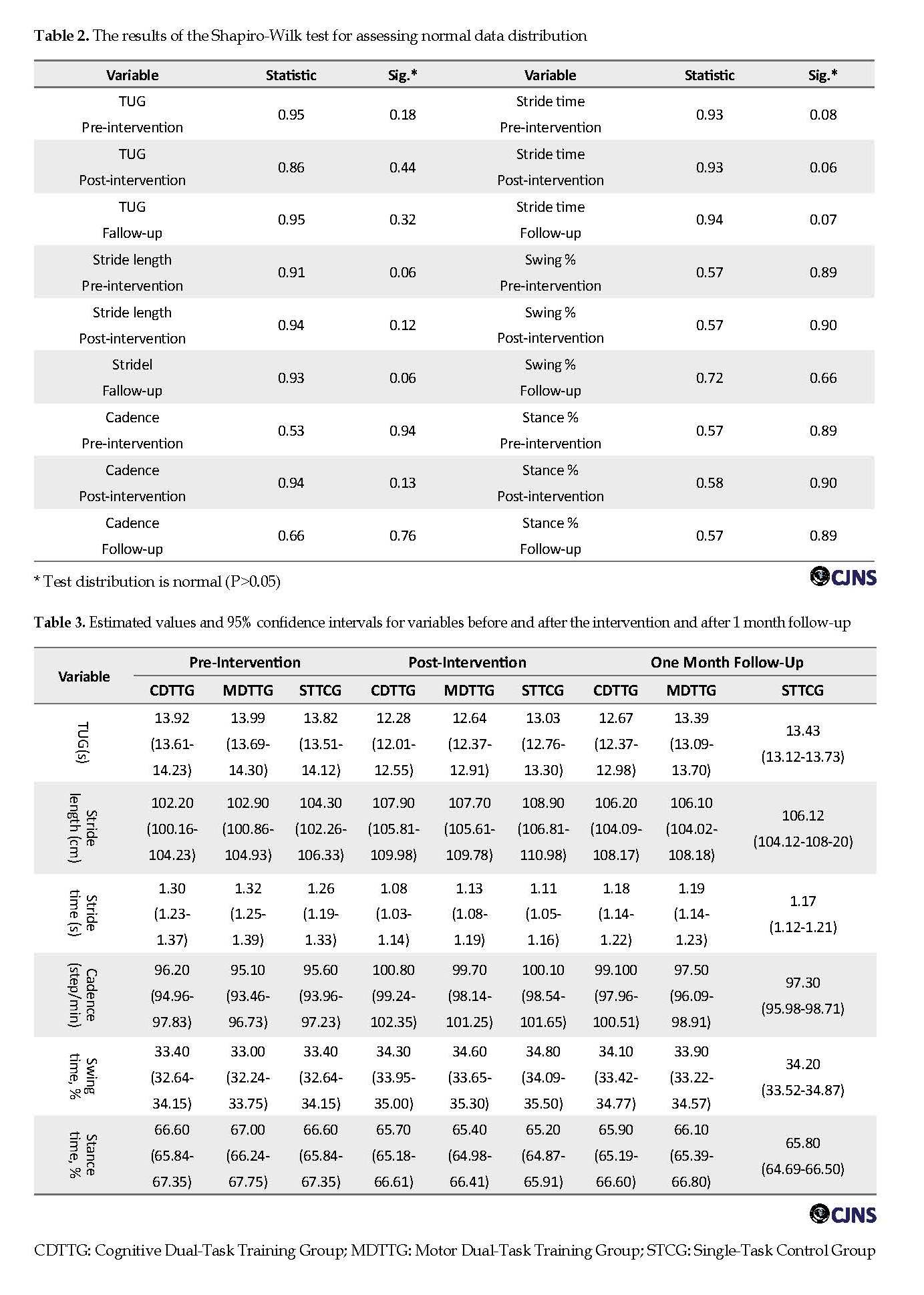
A total number of 32 patients participated in receiving one of training protocols: cognitive DT training (n=11), motor DT training (n=11), or single-Task training (n=10). The dropout rate was 6.2%. One patient dropped out of cognitive DT training and one patient of motor DT training because they could not finish the training protocols. The groups were similar in clinical and demographical variables. Table 1 shows no significant differences in the clinical and demographic variables of subjects at the beginning of the study using One-Way ANOVA (P>0.05). According to Table 2 and based on the Shapiro-Wilk test, all data were normally distributed (P>0.05); therefore parametric statistics were used.
The effect of dual-task and single-task training on outcome measures
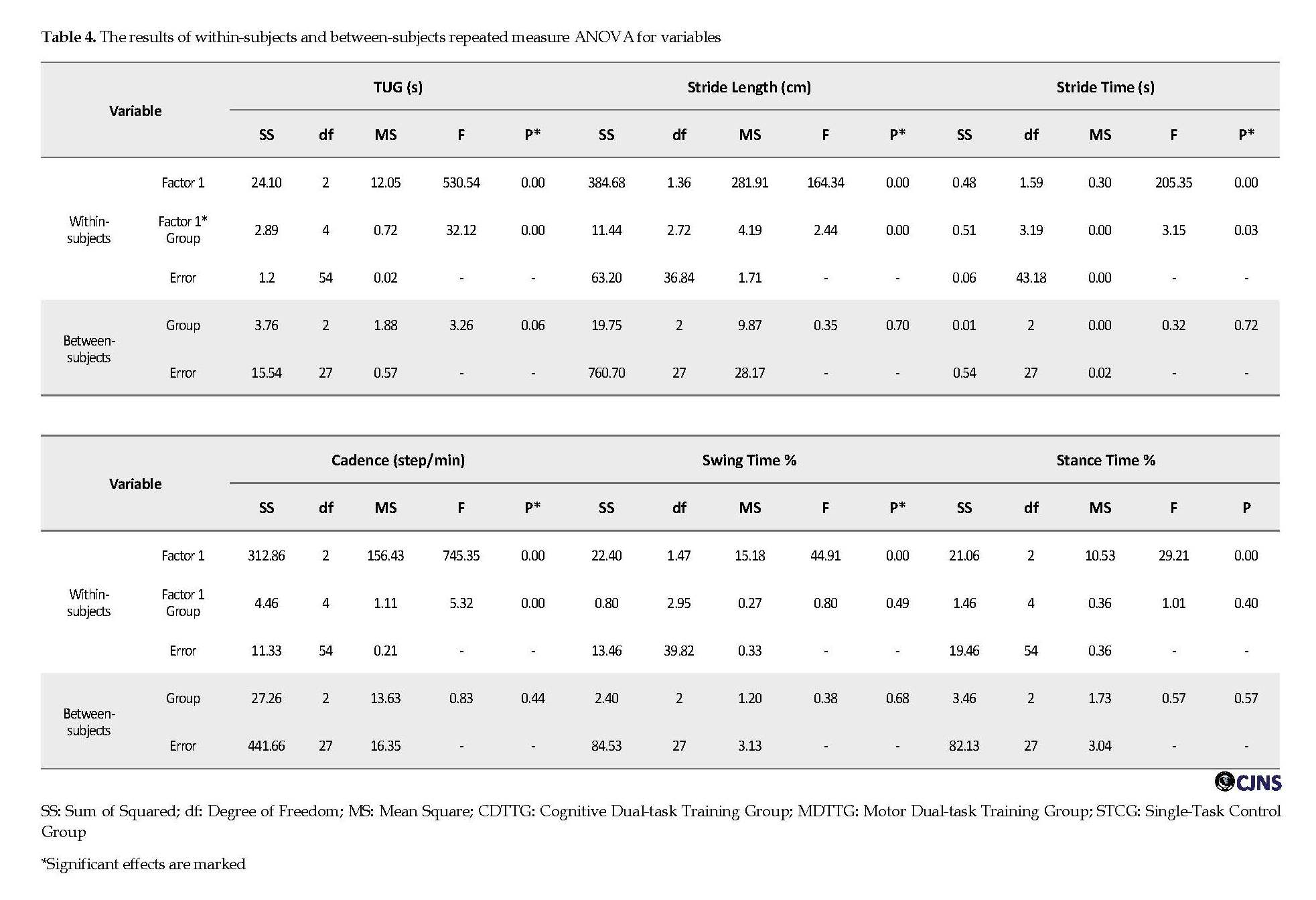
Table 3 presents the estimated values and 95% confidence intervals for variables before and after the intervention and after 1 month follow-up. According to Table 4 and contrary to our hypotheses, no interaction effects were found between “Time” and “Group” for any of the spatiotemporal gait parameters and TUG. It indicates that all training protocols had similar effects (P>0.05).
Timed Up and Go test
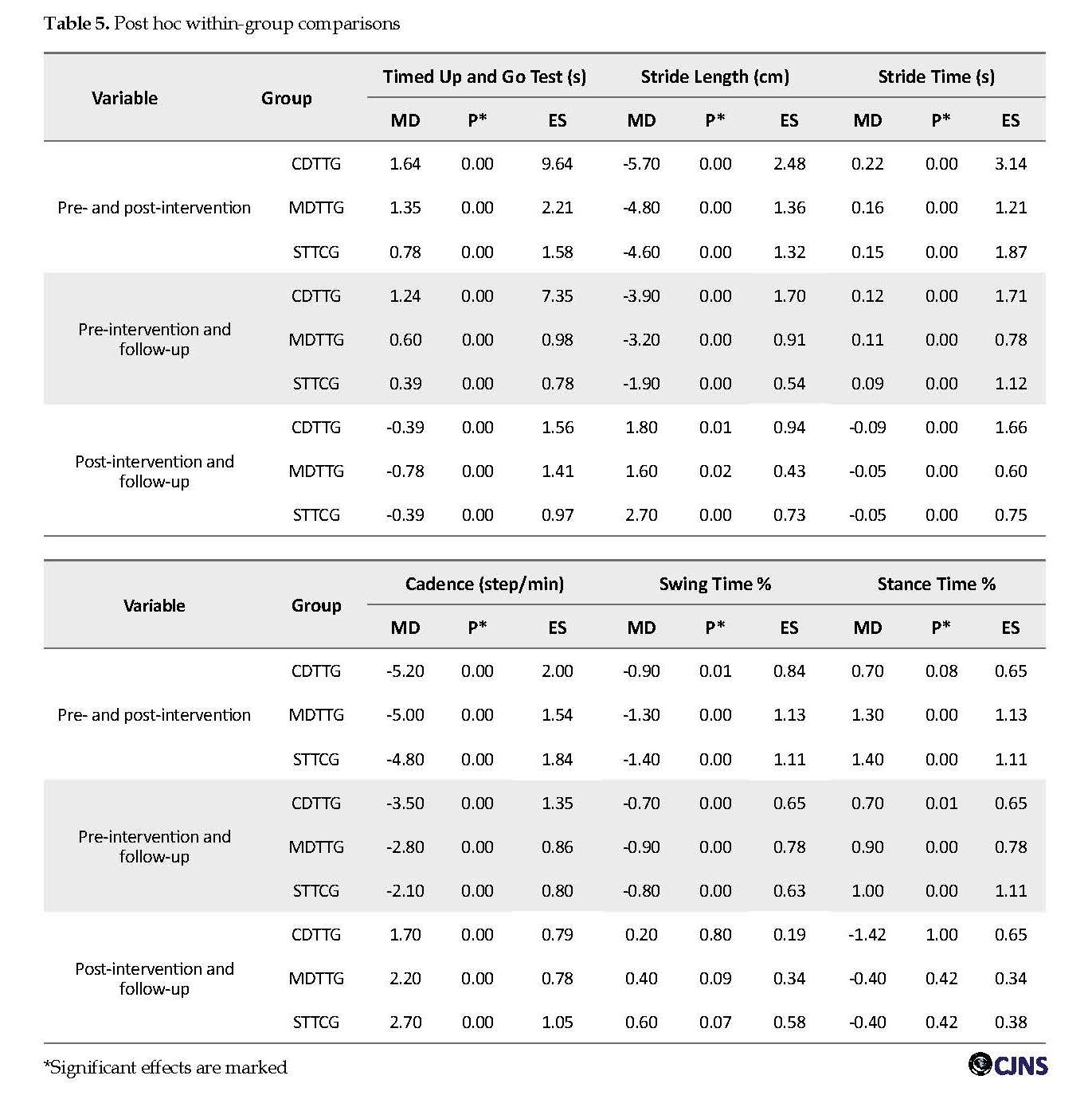
The main effects for “Time” were significant for TUG in within-subjct comparisons in three groups (F=530.54; P=0.00) (Table 4). Post hoc within-group analysis showed significant decreases in time of TUG test after treatment and after one month follow-up in three groups (P≤0.05) (Table 5).
Spatiotemporal gait parameters
The main effects for “Time” were significant for spatiotemporal gait parameters, including stride length (F=164.34; P=0.00), stride time (F=205.35; P=0.00), cadence (F=745.33; P=0.00), swing time % (F=44.91; P=0.00), stance time % (F=29.21; P=0.00) in three groups (Table 4). Post hoc within-group analysis showed a significant increase in stride length and cadence and significant decrease in stride time after intervention and after one month follow-up in three groups (P≤0.05) (Table 5). But for swing time % and stance time %, post hoc comparisons showed significant increase in swing time % and significant decrease in stance time % after the intervention (P≤0.05). These changes were not significant after 1 month follow-up (P>0.05) (Table 5).
Discussion
In this study, we compared the efficacy of two dual-task training programs with a single-Task training program on the improvement of balance and some spatiotemporal gait parameters in people with idiopathic Parkinson Disease. The results of this study indicate that cognitive/ motor dual-task training and single-Task training programs were equally effective in improving balance and some spatiotemporal gait parameters in PD patients. These effects were obvious not only in the single-Task training group but also in the dual-task training groups and maintained for one month after training. Patients with PD suffer from loss of automaticity in movements. Thus, several studies reported balance disorders in patients with PD under DT conditions compared with healthy age-matched control groups [23].
Parkinson disease (PD) is the second most common neurodegenerative disorder after Alzheimer disease that targets basal ganglia [1]. Four million people now live with PD all over the world, and this number will double until 2030 [2]. This illness is characterized by both motor and non-motor symptoms that disrupt activities of daily living [1]. The main motor symptoms include tremor, rigidity, bradykinesia, and postural instability [3]. Patients with PD usually experience cognitive impairments, including deficits in executive function, attention, working memory, and visuospatial domains that can interfere with their mobility [4].
More than half of the patients with PD suffer from gait disturbances depending on their disease severity that causes falling so that between 50% to 68% of people with PD experience at least one fall during a year [5, 6]. Apparently gait impairments like reduced stride length and slower gait velocity are common in patients with PD [7]. Some medications, such as levodopa is the standard treatment for PD, but they become less effective for some symptoms after a long period of usage [5]. Thus, the other kinds of treatments like exercise therapy or physical therapy are needed for these patients.
Dual-Task (DT) performance refers to performing two tasks at the same time. Mobility in daily life frequently requires DT performances, such as talking with carrying a glass of water while walking. Several studies demonstrated some gait disturbances such as increased risk of falling, more freezing of gait, and reduced functional mobility during DT conditions in patients with PD [8-10]. According to the guidelines of physical therapy, DT training is better avoided or used cautiously in PD because its practicality is unclear [11]. Because of loss of automaticity in PD, patients find it challenging to do two tasks at the same time [12]. However, some recent studies showed benefits of DT training in PD patients [13].
Santos et al. in a systematic review, reported that different types of dual-task interventions could improve some gait parameters in patients with PD [3]. As mentioned above, since the people in their activities of daily living face with DT conditions a lot, DT training could be beneficial, especially in patients with balance and gait disorders like PD. Another point is that research on the ability to modify DT performance among people with PD is very limited [14]. Therefore, this study investigates whether cognitive and motor DT training has any effect on balance and some spatiotemporal gait parameters in people with idiopathic PD.
Materials and Methods
Study design
The study was performed with the approval of the University of Guilan and registered in the Iranian Registry of Clinical Trials (No. IRCT20180106038239N1). A single-blind controlled trial was conducted to compare cognitive and motor dual-task training with simple-task trining. The research period included 10 weeks supervised training and one-month follow-up and primary outcomes measured at baseline, after supervised training, and one month later (as follow-up). In this study, the patients were trained and assessed by a physiotherapist in a private clinic of physical therapy. Also, the patients were tested on medications condition, 1-2 hours after taking antiparkinson medications, at the same time of day for pre- and post-intervention and follow up assessments.
Study participants
Considering α=0.05, effect size: 0.5, and analysis power of 0.8, the sample size was estimated as 30 by GPower 3.1 (an excellent freeware program for sample size analysis) [15]. So, thirty patients with mild to moderate PD were recruited for the study. After evaluating the eligibility of the patients, they were informed of the study procedure and signed informed written consent before the study. The patients were assigned to a control group (n=10) and 2 experimental groups (n=20). In experimental groups, there were 10 patients in motor dual-task training group (MDTTG) and 10 patients in cognitive dual-task training group (CDTTG).
Inclusion criteria
Diagnosis of PD by a neurologist, being at stage II-III based on the Hoehn and Yahr scale [16]. Aged between 50 to 75 years. Under stable medication regimen within the previous month and during the period of the research (4 months) [17]. Able to walk 100 m independently without any assistive devices [5].
Exclusion criteria
Having another neurological condition in addition to PD. Suffering from any musculoskeletal or cardiopulmonary conditions that affect the quality of life. Undergoing surgery for PD such as deep brain stimulation. Getting a score of less than 24 in the mini-mental status examination. Having sensory impairment (e.g. blindness, deafness) [5]. Having participated in an organized exercise therapy program in the last 6 months [17].
Outcome measures
In the current study, the outcome measures were timed up and go test (TUG) and some spatiotemporal gait parameters, including stride length (cm), cadence (step/min), stride time (stride/s), swing % (% of gait cycle), and stance % (% of gait cycle). TUG test is a functional test the measures the ability of the patient to rise from a seated position on a chair, walk 3 m, turn, walk back, and sit down. This test requires only a few minutes to accomplish, easy to administer, and requires a few pieces of equipment. In PD, longer TUG test times are associated with decreased mobility. Also, TUG test has a high test-retest reliability and interrater reliability in PD population [18]. For assessing some spatiotemporal gait parameters, HD VideoCam-Kinovea was used.
The motion was simultaneously recorded using two Casio EX-F1 HD VideoCam in the sagittal and frontal plane with the ability to shoot 1920×1080 full HD movies at 60 frames per second. One camera (in the sagittal plane) was placed on a tripod at the height of 50 cm at a distance of 2 m to the center of the pathway to capture a good view of a gait cycle. Another camera was placed in front of the subject at a distance of 3 m [19]. This setting ensured that the calibration area covered the lower limb of the subject (field of view). The outputs (video recordings) from the two HD VideoCam were fed into Kinovea 0.8.25 (an open-source software) to analyze the gait. It seems that HD VideoCam-Kinovea is a reliable motion capture-analysis system (r=0.79). Moreover, it is low cost, portable, and easy to use [20].
Study itervention
In the single-Task training control group (STTCG) and cognitive and motor Dual-Task Training (CDTTG and MDTTG) groups, the patients participated in a 30 session program administered for 45 minutes each session, 3 times per week for 10 weeks. The training was conducted by a physiotherapist in the “on medication” condition (often 1 hour after medication). In the single-Task group, the patients made a selection of exercises presented by Parkinson society of Canada, including wall standing exercise, tandem stance, single-leg stance, standing on toes, squat, march, side bending exercise, trunk rotation exercise, and figure of eight walking [21]. According to overload principle of exercise, training program had a progressive trend.
In the cognitive and motor dual-task groups, the patients did the exercises while performing various additional cognitive or motor tasks. Other cognitive tasks during training included counting backward by 3 s, memory recall, generating category lists (e.g. fruit, sports, names starting with a specific letter) and simple calculation tasks. Additional motor tasks were selected to reflect everyday activities such as doing up buttons, carrying a plate with a glass on top, and transferring coins between pockets or objects like cell phone between hands while training [22]. The patients were instructed to perform additional tasks correctly while doing the exercises.
Statistical analysis
Statistical analysis was performed in SPSS V. 20. The results obtained in the study were considered statistically significant at P≤0.05. For assessing any differences between clinical and demographic variables at the beginning of the study, One-Way analysis of variance (ANOVA) was used. Assumptions of data normality were checked before the analysis by the Shapiro-Wilk test. The data collected from the single-Task group statistically compared with the two dual-task training groups. In this research, for comparison of the effects of single and motor/ cognitive dual-task training on the balance and some spatiotemporal gait parameters, six separate repeated measures ANOVA were conducted. Also, Greenhouse-Geisser correction was used when the obtained results from the Mauchly’s test of sphericity indicated a violation of the sphericity assumption (P≤0.05). Post hoc analyses were carried out using Bonferroni corrections for multiple comparisons.
Results
A total number of 32 patients participated in receiving one of training protocols: cognitive DT training (n=11), motor DT training (n=11), or single-Task training (n=10). The dropout rate was 6.2%. One patient dropped out of cognitive DT training and one patient of motor DT training because they could not finish the training protocols. The groups were similar in clinical and demographical variables. Table 1 shows no significant differences in the clinical and demographic variables of subjects at the beginning of the study using One-Way ANOVA (P>0.05). According to Table 2 and based on the Shapiro-Wilk test, all data were normally distributed (P>0.05); therefore parametric statistics were used.
The effect of dual-task and single-task training on outcome measures
Table 3 presents the estimated values and 95% confidence intervals for variables before and after the intervention and after 1 month follow-up. According to Table 4 and contrary to our hypotheses, no interaction effects were found between “Time” and “Group” for any of the spatiotemporal gait parameters and TUG. It indicates that all training protocols had similar effects (P>0.05).
Timed Up and Go test
The main effects for “Time” were significant for TUG in within-subjct comparisons in three groups (F=530.54; P=0.00) (Table 4). Post hoc within-group analysis showed significant decreases in time of TUG test after treatment and after one month follow-up in three groups (P≤0.05) (Table 5).
Spatiotemporal gait parameters
The main effects for “Time” were significant for spatiotemporal gait parameters, including stride length (F=164.34; P=0.00), stride time (F=205.35; P=0.00), cadence (F=745.33; P=0.00), swing time % (F=44.91; P=0.00), stance time % (F=29.21; P=0.00) in three groups (Table 4). Post hoc within-group analysis showed a significant increase in stride length and cadence and significant decrease in stride time after intervention and after one month follow-up in three groups (P≤0.05) (Table 5). But for swing time % and stance time %, post hoc comparisons showed significant increase in swing time % and significant decrease in stance time % after the intervention (P≤0.05). These changes were not significant after 1 month follow-up (P>0.05) (Table 5).
Discussion
In this study, we compared the efficacy of two dual-task training programs with a single-Task training program on the improvement of balance and some spatiotemporal gait parameters in people with idiopathic Parkinson Disease. The results of this study indicate that cognitive/ motor dual-task training and single-Task training programs were equally effective in improving balance and some spatiotemporal gait parameters in PD patients. These effects were obvious not only in the single-Task training group but also in the dual-task training groups and maintained for one month after training. Patients with PD suffer from loss of automaticity in movements. Thus, several studies reported balance disorders in patients with PD under DT conditions compared with healthy age-matched control groups [23].
According to the evidence-based rehabilitation guidelines in PD, it is better to avoid DT situations and divide complex tasks into easier subcomponents [24]. In PD patients, frontoparietal circuits showed greater activation during DT activities compared with single-Task performance, and flexible internetwork compensation may be hampered. Besides the structural limitations, changing task prioritization and allocating attention to the first task leads to difficulty in second task performance [14]. But in recent years, European guideline provides a different opinion, stating that in Hoehn and Yahr stages II and III, DT training may be safe and effective [25]. The findings of this study showed that cognitive/ motor dual-task training and single-Task training could improve TUG and this improvement would remain even after 1 month follow-up. In agreement with our findings, Romenets et al. reported significant improvements in TUG and DT-TUG performance compared to the control group after 12 weeks Tango dancing [26]. In spite of different types of DT training, the effect of training on TUG was similar in these studies.
Regarding gait analysis, improvement in gait velocity can result from increased cadence, increased stride length, or both. The results of our study revealed that stride length and cadence improved with both single and dual-task training. As we expected, significant effects were also observed for other spatiotemporal gait parameters that are strictly related to gait velocity, stride length, and cadence.
For example, we observed a significant decrease in stride time and stance time percentage. In another study, Strouwen et al. compared the efficacy of integrated dual-task training and consecutive dual-task training on gait parameters and risk of fall in patients with PD. They concluded that consecutive and integrated dual-task training could lead to similar improvements in dual-task gait velocity without increasing fall risk. Therefore, these findings support the application of dual-task training in clinical practice [11]. A dual task directs the performer’s attention toward an external source of attention, while performing a primary task. As stated in constrained action hypothesis, this attentional change might allow motor systems to function automatically, resulting in more effective performance [27].
In contrast to our hypothesis, no significant difference was found between study groups. In the current research, even the control group was trained. It seems that the lack of an actual control group without any intervention can be a reason for achieving these results. As reported by studies of motor learning, the effect of DT training will transfer not only to the DT performance but also to the single-Task performance [14]. That could be another reason for lack of any significant difference between (Single-Task control group) STCG and DT training groups in our study. Although we used GPower software for calculating sample size, choosing a small sample size is another limitation of this research, which can affect the study results.
Conclusion
Single task and motor/cognitive dual-task training were equally effective in improvement of balance and some spatiotemporal gait parameters in people with PD. The positive effects retained for one month that was indicative of motor learning capacity in PD. Contrary to current belief, DT training is not hazardous. Thus, DT training should be included in rehabilitation programs by physiotherapists in their clinical practice.
Ethical Considerations
Compliance with ethical guidelines
The study protocol was approved by the Ethics Committee of Guilan University of Medical Sciences (No.IR.GUMS.REC.1396.381), registered at the Iranian Registry of Clinical Trials (IRCT20180106038239N1) and conducted after obtaining the necessary permissions. All the study procedures were in compliance with the ethical guidelines of the Declaration of Helsinki 2013.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
Drafting the paper: Tahereh Pourkhani, Hassan Daneshmandi, Ali Asghar Norasteh; Data collection: Tahereh Pourkhani; Writing the review and editing: Tahereh Pourkhani, Hassan Daneshmandi, Ali Asghar Norasteh; Resources: Tahereh Pourkhani, Hassan Daneshmandi, Ali Asghar Norasteh, Babak Bakhshayesh Eghbali, Parisa Sedaghati; Supervision: Hassan Daneshmandi, Ali Asghar Norasteh, Babak Bakhshayesh Eghbali, Parisa Sedaghati.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
The authors express sincere gratitude toward the personnel of The University of Guilan and Guilan University of Medical Science for their dedicated support.
References
Schapira AHV, Olanow CW. Neuroprotection in Parkinson’s disease: Mysteries, myths, and misconceptions. JAMA. 2004; 291(3):358-64. [DOI:10.1001/jama.291.3.358] [PMID]
Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007; 68(5):384-6. [DOI:10.1212/01.wnl.0000247740.47667.03] [PMID]
Santos AAL, Campos C, Bento T, Lattari E, Egidio Nardi A, Barbosa F, et al. Effects of dual-task interventions on gait performance of patients with Parkinson’s disease: A systematic review. Med Express. 2016; 3(4):1-10. [DOI:10.5935/MedicalExpress.2016.04.01]
Peterson DS, King LA, Cohen RG, Horak FB. Cognitive contributions to freezing of gait in Parkinson disease: Implications for physical rehabilitation. Phys Ther. 2016. 96(5):659-70. [DOI:10.2522/ptj.20140603] [PMID] [PMCID]
Brauer SG, Woollacott MH, Lamont R, Brauer SG, Woollacott MH, Lamont R, et al. Single and dual task gait training in people with Parkinson’s disease: A protocol for a randomised controlled trial. BMC Neurol. 2011; 11:90. [DOI:10.1186/1471-2377-11-90] [PMID] [PMCID]
Ashburn A, Stack E, Ballinger C, Fazakarley L, Fitton C. The circumstances of falls among people with Parkinson’s disease and the use of falls diaries to facilitate reporting. Disabil Rehabil. 2008; 30(16):1205-12. [DOI:10.1080/09638280701828930] [PMID]
Lord S, Galna B, Rochester L. Moving forward on gait measurement: toward a more refined approach. Mov Disord. 2013; 28(11):1534-43. [DOI:10.1002/mds.25545] [PMID]
Heinzel S, Maechtel M, Hasmann SE, Hobert MA, Heger T, Berg D, et al. Motor dual-tasking deficits predict falls in Parkinson’s Disease: A prospective study. Parkinsonism Relat Disord. 2016; 26:73-7. [DOI:10.1016/j.parkreldis.2016.03.007] [PMID]
Spildooren J, Vercruysse S, Desloovere K, Vandenberghe W, Kerckhofs E, Nieuwboer A. Freezing of gait in Parkinson’s Disease: The impact of dual-tasking and turning. Mov Disord. 2010; 25(15):2563-70. [DOI:10.1002/mds.23327] [PMID]
Fuller RL, Van Winkle EP, Anderson KE. Dual task performance in Parkinson’s Disease: A sensitive predictor of impairment and disability. Parkinsonism Relat Disord. 2013; 19(3):325-8. [DOI:10.1016/j.parkreldis.2012.11.011] [PMID]
Strouwen C, Molenaar EALM, Munks L, Keus SHJ, Zijlmans JCM, Vandenberghe W, et al. Training Dual Tasks Together or apart in Parkinson’s Disease: Results From the DUALITY trial. Mov Disord. 2017; 32(8):1201-10. [DOI:10.1002/mds.27014] [PMID]
Wu T, Chan P, Hallett M. Effective connectivity of neural networks in automatic movements in Parkinson’s Disease. Neuroimage. 2010; 49(3):2581-7. [DOI:10.1016/j.neuroimage.2009.10.051] [PMID] [PMCID]
De Freitas TB, Leite PHW, Dona F, Pompeu JE, Swarowsky A, Torriani-Pasin C. The effects of dual task gait and balance training in Parkinson’s Disease: A systematic review. Physiother Theory Pract. 2018; 3:1-9. [DOI:10.1080/09593985.2018.1551455] [PMID]
Kelly VE, Eusterbrock AJ, Shumway-Cook A. The effects of instructionson dual-task walking and cognitive task performance in people with Parkinson’s Disease. Parkinsons Dis. 2012; 2012:1-9. [DOI:10.1155/2012/671261] [PMID] [PMCID]
Cunningham JB, McCrum-Gardner E, Gardner E. Power, effect and sample size using GPower: Practical issues for researchers and members of research ethics committees. Evid Based Midwifery. 2007; 5(4):132-7.
van den Heuvel MRC, Kwakkel G, Beek PJ, Berendse HW, Daffertshofer A, van Wegen EE. Effects of augmented visual feedback during balance training in Parkinson’s Disease: A pilot randomized clinical trial. Parkinsonism Relat Disord. 2014; 2014(43):1352-8. [DOI:10.1016/j.parkreldis.2014.09.022] [PMID]
Yang Y, Hao Y, Tian W, Yang Y, Hao YL, Tian WJ, et al. The effectiveness of Tai Chi for patients with Parkinson’s Disease: Study protocol for a randomized controlled trial. Bio Med Central. 2015; 16:111. [DOI:10.1186/s13063-015-0639-8] [PMID] [PMCID]
Nocera JR, Stegemöller EL, Malaty IA, Okun MS, Marsiske M, Hass CJ, et al. Using the Timed Up & Go Test in a clinical setting to predict falling in Parkinson’s Disease. Arch Phys Med Rehabil. 2013; 94(7):1300-5. [DOI:10.1016/j.apmr.2013.02.020] [PMID] [PMCID]
Fonda B, Sarabon N, Li FX. Validity and reliability of different kinematics methods used for bike fitting. J Sports Sci. 2014; 32(10):40-96. [DOI:10.1080/02640414.2013.868919] [PMID]
Sanudo B, Rueda D, del Pozo-Cruz B, Carrasco L. Validation of A video analysis software package for quantifying movement velocity in resistance exercises. J Strength Cond Res. 2016; 30(10):2934-41. [DOI:10.1519/JSC.0000000000000563] [PMID]
Sacheli MA, Murray DK, Vafai N. Habitual exercisers versus sedentary subjects with Parkinson’s Disease: Multimodal PET and fMRI study. Move Disord. 2018; 32(12):1945-50. [DOI:10.1002/mds.27498] [PMID]
Canning GC, Ada L, Woodhouse E. Multiple-task walking training in people with mild to moderate Parkinson’s Disease: A pilot study. Clin Rehabil. 2008; 22(3):226-33. [DOI:10.1177/0269215507082341] [PMID]
Wild LB, de Lima DB, Balardin JB. Freezing of gait in Parkinson’s Disease: The impact of dual-tasking and turning. Mov Disord. 2013; 25(15):2563-70 [DOI:10.1002/mds.23327] [PMID]
Keus SH, Bloem BR, Hendriks EJ. Evidence-based analysis of physical therapy in Parkinson’s Disease with recommendations for practice and research. Move Disord. 2007; 22(4):451-60. [DOI:10.1002/mds.21244] [PMID]
Keus S, Munneke M, Graziano M, Paltamaa J, Pelosin E, Domingos J, et al. European physiotherapy guideline for Parkinson’s Disease. The Netherlands: KNGF/ParkinsonNet; 2014.
Rios Romenets S, Anang J, Fereshtehnejad SM, Pelletier A, Postuma R. Tango for treatment of motor and non-motor manifestations in Parkinson’s Disease: Randomized control study. Complement Ther Med. 2015; 23(2):175-84. [DOI:10.1016/j.ctim.2015.01.015] [PMID]
Ghai S, Ghai I, Effenberg A. Effects of dual tasks and dual-task training on postural stability: A systematic review and meta-analysis. Clin Interv Aging. 2017; 12:557-77. [DOI:10.2147/CIA.S125201] [PMID] [PMCID]
Type of Study: Research |
Subject:
Special
Received: 2019/02/19 | Accepted: 2019/07/20 | Published: 2019/10/1
Received: 2019/02/19 | Accepted: 2019/07/20 | Published: 2019/10/1
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |