Fri, Apr 26, 2024
Volume 5, Issue 1 (Winter 2019)
Caspian J Neurol Sci 2019, 5(1): 28-33 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Alijani B, Ghadarjani S, Naseri A, Zamanidoost M R. Infected External Ventricular Drainage After Ruptured Intracranial Aneurysm: Clipping Time Challenge. Caspian J Neurol Sci 2019; 5 (1) :28-33
URL: http://cjns.gums.ac.ir/article-1-262-en.html
URL: http://cjns.gums.ac.ir/article-1-262-en.html
Infected External Ventricular Drainage After Ruptured Intracranial Aneurysm: Clipping Time Challenge
1- Department of Neurosurgery, Poursina Hospital, Guilan University of Medical Sciences, Guilan, Iran
Full-Text [PDF 1039 kb]
(723 Downloads)
| Abstract (HTML) (2021 Views)
Full-Text: (724 Views)
Introduction
Amajor cause of morbidity and mortality is aneurysmal Subarachnoid Hemorrhage (aSAH) that hospitalize over 24800 people every year [1].
Although aSAH is responsible for only 5% of strokes, it accounts for 25% of stroke-related deaths all over the world [2]. A well-known complication of subarachnoid hemorrhage (SAH) is acute hydrocephalus that leads to more neurological deficits and mortality than SAH alone [3]. The incidence of acute hydrocephalus after SAH is 20% which may require emergency external ventricular drainage (EVD) placement as a lifesaving measure to relieve acute hydrocephalus and decrease intracranial pressure (ICP) [4, 5]. To watch over possible ICP and treat secondary hydrocephalus, the treatment choice is usually inserting ventriculostomy catheters. However, this procedure may increase the risk of infection, because microorganisms can infect the catheter and spread via cerebrospinal fluid (CSF) to meninges and brain causing meningitis or ventriculitis. Thus, these infections are called ventriculostomy-related infections (VRIs) [6].
Bacterial ventriculitis has been reported in 3%–29% of SAH patients [7] and are strongly correlated to the placement of CSF catheters [8]. Risk factors for infection comprise non-sterile insertion techniques, number of catheterizations, and catheter placement duration [9, 10]. The treatment usually consists of antibiotics administration via intravenous, intrathecal, or combined dual-route [11].
Three days following aSAH is widely regarded as the acceptable treatment window according to early results from the International Cooperative Study on the Timing of Aneurysm Surgery. Early treatment is associated with decreased chances of re-rupture and overall mortality while allowing for more aggressive management of potential complications such as cerebral vasospasm [12]. The simultaneous management of brain aneurysm and infection induced by EVD can be challenging. Considering the risk of placement of a metal clip in an infected environment, the timing of clipping in these patients is important. So far, limited studies have been conducted to clarify which choice of treatment is better; to ligate the aneurysm in the presence of infection or to wait for infection clearance.
We performed a retrospective study on the treatment of patients with ruptured aneurysm that simultaneously had EVD-induced infection and discussed the outcomes.
Materials and Methods
This study was conducted on 42 consecutive patients with spontaneous SAH who were treated with EVD. They were retrospectively screened and all of them were treated at the Department of Neurosurgery, Poursina Hospital, affiliated to Guilan University of Medical Sciences between January 2016 and December 2018. The patients were admitted to the neuro-ICU when the diagnosis of SAH verified by brain CT Scans.
The acute symptomatic hydrocephalus was diagnosed based on CT criteria using the bi-caudate index (width of the frontal horns at the level of the caudate nuclei divided by the width of the parenchyma at the same level >95th percentile for age) and significant clinical symptoms (e.g., gradual decline of consciousness level, constricted unreactive pupils with preservation of all other brainstem reflexes, and upward gaze impairment) within acute period of the SAH onset [12]. Placement and maintenance of the external ventricular drainage were performed according to standardized protocols to minimize the infection risk. The external ventricular catheter was placed under a sterile condition in the operating room. The surgeons used standard external ventricular catheters without antibiotic or silver impregnation. The catheter was inserted through a frontal lobe burr hole and fixated with sutures after at least 5 cm subcutaneous tunneling [13].
EVD implantation was performed under strictly aseptic conditions and antibiotic prophylaxis (a single shot of 1 g ceftriaxone intravenously) in the operating room. Ventriculostomy system was a closed one and CSF samples would be collected if clinical signs of infection such as fever, unexplained rise in system inflammatory parameters, or deteriorating neurological performance were seen. The catheters were usually kept there. The bleeding source was identified by computed tomography angiography (CTA). We excluded patients that did not suffer VRIs and had normal CTA.
The study information was extracted from the patient’s electronic records regarding age, gender, neurological grading of Hunt and Hess on admission, length of EVD treatment, results of laboratory tests of CSF (proven or suspected ventriculitis) and time period from ictus to aneurysm surgical clipping, time period from infection to aneurysm surgical clipping, length of VRIs, and patients’ outcomes.
EVD-associated ventriculitis was considered “proven ventriculitis” after a positive microbiological CSF culture along with clinical signs of infection, or “suspected ventriculitis” due to abnormal CSF parameters like low CSF glucose level (<40 mg/dL, or <50% of serum glucose), high CSF protein level (>50 mg/dL), CSF pleocytosis (100/mm), positive CSF Gram-stain and organism cultured from the blood or EVD tip in the absence of a positive CSF culture. These criteria are obtained from the nosocomial infections criteria issued by the Centers for Disease Control and Prevention (CDC) for diagnosing EVD-associated ventriculitis of the year 2008 [14, 15].
Statistical analysis
A retrospective review of patients’ records was performed for all who underwent EVD placement. Their demographic data (sex, age, and indication for EVD insertion), age, gender, time of bleeding, time of EVD placement, length of EVD treatment and time of aneurysm clipping, time from infection to aneurysm clipping, length of VRIs and patients’ outcome and results
The Analysis of Variance (ANOVA) was used to compare study quantitative variables, based on outcome and results. Non-parametric Kruskal-Wallis test was used for non-normality distributed variables. Fisher exact test and Chi-square test were used to compare the outcome based on VRIs as well as the time period from ictus to aneurysm clipping. All analyses were done in SPSS version 20. P values of less than 0.05 were considered to be significant.
Results
A total of 42 patients with a primary diagnosis of aSAH who underwent EVD placement because of hydrocephalus were included in the study. All of these patients underwent aneurysm clipping. A total of 16(38%) patients were male and 26(62%) were females.
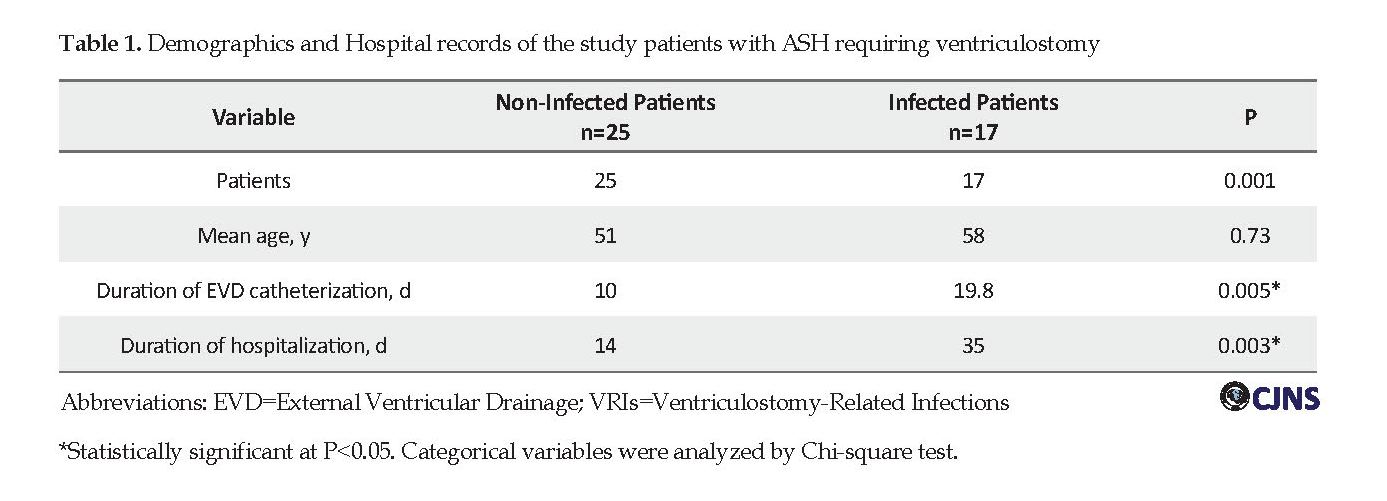
Classification of patients based on Hunt and Hess neurological grading at the admission time revealed that 18(42.9%) patients were Grade ΙΙ, 12(28.6%) Grade ΙΙΙ, 8(19%) Grade ΙV and 4(9.5%) Grade V. 17(40.4%) patients got complicated with ventriculitis (5 [11.9%] patients had proven ventriculitis and 12[28.6%] patients had suspected ventriculitis) and 25(59.6%) patients were managed without VRIs (P=0.001). The patients’ demographic data are presented in Table 1.
The mean duration between EVD placement and onset of infection was 3.4 days (range: 2-5 days). The mean duration of EVD catheterization in non-infected patients was 10 days and the mean hospital stay in this group was 14 days.
Amajor cause of morbidity and mortality is aneurysmal Subarachnoid Hemorrhage (aSAH) that hospitalize over 24800 people every year [1].
Although aSAH is responsible for only 5% of strokes, it accounts for 25% of stroke-related deaths all over the world [2]. A well-known complication of subarachnoid hemorrhage (SAH) is acute hydrocephalus that leads to more neurological deficits and mortality than SAH alone [3]. The incidence of acute hydrocephalus after SAH is 20% which may require emergency external ventricular drainage (EVD) placement as a lifesaving measure to relieve acute hydrocephalus and decrease intracranial pressure (ICP) [4, 5]. To watch over possible ICP and treat secondary hydrocephalus, the treatment choice is usually inserting ventriculostomy catheters. However, this procedure may increase the risk of infection, because microorganisms can infect the catheter and spread via cerebrospinal fluid (CSF) to meninges and brain causing meningitis or ventriculitis. Thus, these infections are called ventriculostomy-related infections (VRIs) [6].
Bacterial ventriculitis has been reported in 3%–29% of SAH patients [7] and are strongly correlated to the placement of CSF catheters [8]. Risk factors for infection comprise non-sterile insertion techniques, number of catheterizations, and catheter placement duration [9, 10]. The treatment usually consists of antibiotics administration via intravenous, intrathecal, or combined dual-route [11].
Three days following aSAH is widely regarded as the acceptable treatment window according to early results from the International Cooperative Study on the Timing of Aneurysm Surgery. Early treatment is associated with decreased chances of re-rupture and overall mortality while allowing for more aggressive management of potential complications such as cerebral vasospasm [12]. The simultaneous management of brain aneurysm and infection induced by EVD can be challenging. Considering the risk of placement of a metal clip in an infected environment, the timing of clipping in these patients is important. So far, limited studies have been conducted to clarify which choice of treatment is better; to ligate the aneurysm in the presence of infection or to wait for infection clearance.
We performed a retrospective study on the treatment of patients with ruptured aneurysm that simultaneously had EVD-induced infection and discussed the outcomes.
Materials and Methods
This study was conducted on 42 consecutive patients with spontaneous SAH who were treated with EVD. They were retrospectively screened and all of them were treated at the Department of Neurosurgery, Poursina Hospital, affiliated to Guilan University of Medical Sciences between January 2016 and December 2018. The patients were admitted to the neuro-ICU when the diagnosis of SAH verified by brain CT Scans.
The acute symptomatic hydrocephalus was diagnosed based on CT criteria using the bi-caudate index (width of the frontal horns at the level of the caudate nuclei divided by the width of the parenchyma at the same level >95th percentile for age) and significant clinical symptoms (e.g., gradual decline of consciousness level, constricted unreactive pupils with preservation of all other brainstem reflexes, and upward gaze impairment) within acute period of the SAH onset [12]. Placement and maintenance of the external ventricular drainage were performed according to standardized protocols to minimize the infection risk. The external ventricular catheter was placed under a sterile condition in the operating room. The surgeons used standard external ventricular catheters without antibiotic or silver impregnation. The catheter was inserted through a frontal lobe burr hole and fixated with sutures after at least 5 cm subcutaneous tunneling [13].
EVD implantation was performed under strictly aseptic conditions and antibiotic prophylaxis (a single shot of 1 g ceftriaxone intravenously) in the operating room. Ventriculostomy system was a closed one and CSF samples would be collected if clinical signs of infection such as fever, unexplained rise in system inflammatory parameters, or deteriorating neurological performance were seen. The catheters were usually kept there. The bleeding source was identified by computed tomography angiography (CTA). We excluded patients that did not suffer VRIs and had normal CTA.
The study information was extracted from the patient’s electronic records regarding age, gender, neurological grading of Hunt and Hess on admission, length of EVD treatment, results of laboratory tests of CSF (proven or suspected ventriculitis) and time period from ictus to aneurysm surgical clipping, time period from infection to aneurysm surgical clipping, length of VRIs, and patients’ outcomes.
EVD-associated ventriculitis was considered “proven ventriculitis” after a positive microbiological CSF culture along with clinical signs of infection, or “suspected ventriculitis” due to abnormal CSF parameters like low CSF glucose level (<40 mg/dL, or <50% of serum glucose), high CSF protein level (>50 mg/dL), CSF pleocytosis (100/mm), positive CSF Gram-stain and organism cultured from the blood or EVD tip in the absence of a positive CSF culture. These criteria are obtained from the nosocomial infections criteria issued by the Centers for Disease Control and Prevention (CDC) for diagnosing EVD-associated ventriculitis of the year 2008 [14, 15].
Statistical analysis
A retrospective review of patients’ records was performed for all who underwent EVD placement. Their demographic data (sex, age, and indication for EVD insertion), age, gender, time of bleeding, time of EVD placement, length of EVD treatment and time of aneurysm clipping, time from infection to aneurysm clipping, length of VRIs and patients’ outcome and results
The Analysis of Variance (ANOVA) was used to compare study quantitative variables, based on outcome and results. Non-parametric Kruskal-Wallis test was used for non-normality distributed variables. Fisher exact test and Chi-square test were used to compare the outcome based on VRIs as well as the time period from ictus to aneurysm clipping. All analyses were done in SPSS version 20. P values of less than 0.05 were considered to be significant.
Results
A total of 42 patients with a primary diagnosis of aSAH who underwent EVD placement because of hydrocephalus were included in the study. All of these patients underwent aneurysm clipping. A total of 16(38%) patients were male and 26(62%) were females.
Classification of patients based on Hunt and Hess neurological grading at the admission time revealed that 18(42.9%) patients were Grade ΙΙ, 12(28.6%) Grade ΙΙΙ, 8(19%) Grade ΙV and 4(9.5%) Grade V. 17(40.4%) patients got complicated with ventriculitis (5 [11.9%] patients had proven ventriculitis and 12[28.6%] patients had suspected ventriculitis) and 25(59.6%) patients were managed without VRIs (P=0.001). The patients’ demographic data are presented in Table 1.
The mean duration between EVD placement and onset of infection was 3.4 days (range: 2-5 days). The mean duration of EVD catheterization in non-infected patients was 10 days and the mean hospital stay in this group was 14 days.
The mean duration of EVD catheterization in infected patients was 19.8 days that the minimum duration was 12 days and the maximum was 30 days and the mean time of hospital stay in this group was 35 days.
Duration of EVD catheterization in infected patients was greater than that in non-infected patients (19.8 days compared with 10 days) (P=0.005). The mean time of hospital stay in patients with EVD infections was also significantly longer than that in the patients without infections (35 days vs. 14 days, P=0.003). All of the patients underwent open surgery aneurysm clipping. Thirteen (12.5%) patients in the first 3 days and 20(62.5%) patients on days 4-14 and 8(25%) patients after 2 weeks underwent surgery.
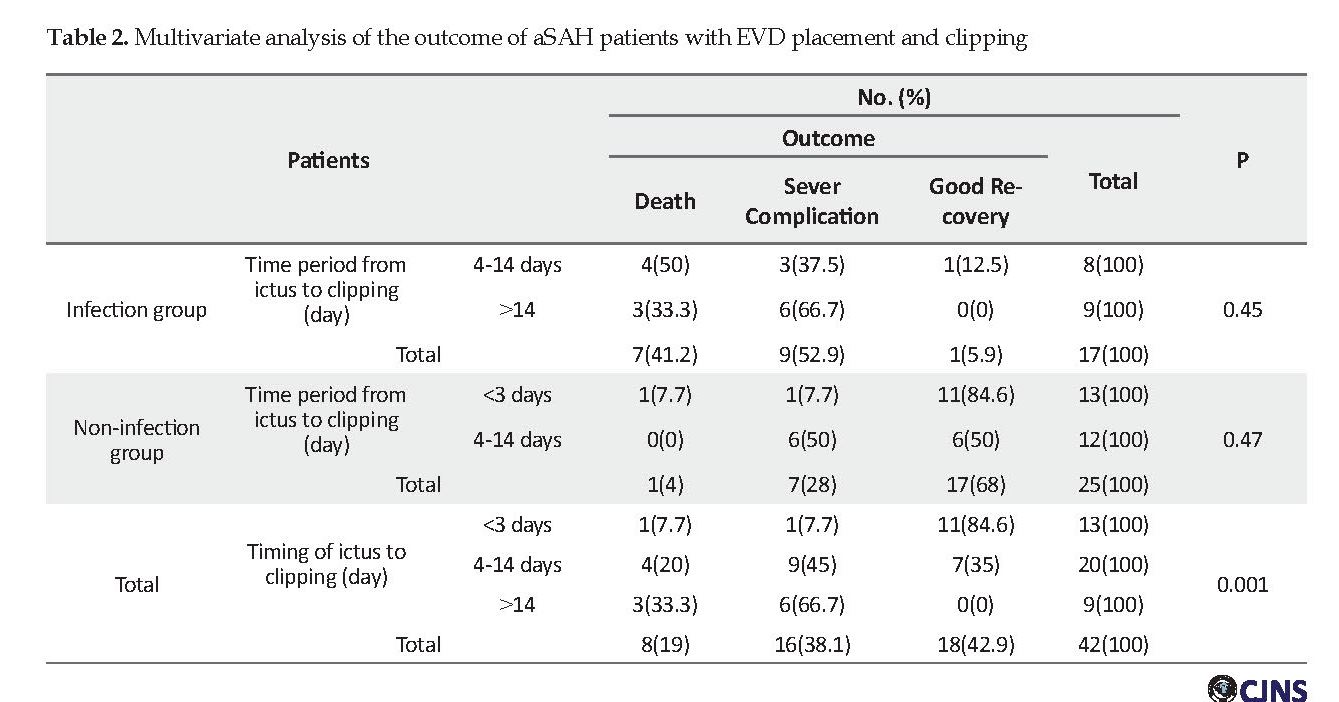
In this study, we categorized the time period from ictus to aneurysm clipping into three time categories of less than 3 days, 4-14 days, and more than 14 days. In the infection group, 8 patients underwent clipping within 4-14 days and 9 patients after 14 days. In the non-infection group, 13 patients underwent clipping in less than 3 days, and 12 patients in 4-14 days and no patient underwent surgery after 14 days. The mean duration from infection to aneurysm surgical clipping in the infection group was 7.5 days (range: 5-10 days).
Postoperative complications such as morbidity and mortality were evaluated in all patients. Among non-infected patients, 68% of the patients had a good recovery, 4% died and 28% developed severe complications and in non-infected patients who underwent surgery in less 3 days, 84.6% had a good recovery and 7.7% developed severe complications. With regard to the outcome, the best time of clipping is less than 3 days with the least complications (P=0.047).
Among the infected patients, 41.2% of the patients died and 52.9% developed severe complications and 5.9% had a good recovery. In the infected patients who underwent surgery in 4-14 days, 12.5% had a good recovery and 37.5% developed severe complications and 50% of patients died. So the rate of death in patients with surgery in 4-14 days was more than patients with surgery after 2 weeks and the rate of severe complications was more after 2 weeks (Table 2). Multivariate logistic regression analysis showed a significant association between the factors involved in severe complications and postoperative death in patients with VRIs (Table 2).
Discussion
CSF diversion such as EVD is the standard treatment for SAH-induced hydrocephalus. VRIs have been investigated extensively in many studies and infection rates of 0% to 22% and many of risk factors, including SAH, IVH, craniotomy, systemic infection, and longer duration of EVD have been reported [9, 16]. This retrospective analysis found that VRIs rate was 40.4%, which is slightly more than other previous studies. This study indicates that duration of EVD insertion and duration of hospital stay are associated with EVD infection, a finding consistent with several studies in the literature [17-19].
Since the advent of early microsurgical and endovascular approaches to aneurysm treatment, the risk of aneurysm re-rupture has reduced. Timely repair of the ruptured aneurysm by either endovascular coiling or open surgery clipping is a critical element in the effective management of the patients with SAH [20, 21]. Early aneurysm repair in less than 3 days reduces the risk of rebreeding, allows for aggressive management of vasospasm, and relieves restrictions on mobilization and physical therapy, thus resulting in reduced morbidity and mortality compared with the patients receiving delayed surgery [22, 23]. Therefore aneurysm clipping in less 3 days is associated with less morbidity and mortality. Based on our study on the evaluation of non-infected patients, clipping in this time is accompanied by a good outcome. And in the infection group, the aneurysm clipping later than 4 day results in high mortality and morbidity rate.
Among the infected patients, 41.2% died and 52.9% developed severe complications and 5.9% had a good recovery. In the infected patients who underwent surgery between 4 and 14 days, 12.5% had a good recovery and 37.5% developed severe complications and 50% died, with no significant difference with those who underwent surgery after 14 days. Since the in the non-infection group, no patient underwent surgical clipping in less than 3 days and given that the most complications and risk of vasospasm happen between 4 and 14 days, it is better that infected patients undergo surgical clipping after this time or after the recovery of VRIs. However, the time of aneurysm treatment and surgical procedure approach in patients with VRIs have not been determined. This study showed that the placement of a metal clip in the infected environment increases the duration of infection and the length of stay in the hospital and result in poor outcome.
This analysis shows the increased rate of moderate to severe neurological deficits among patients receiving aneurysm clipping during the infection period. So this challenge needs to be further studied. Whether to perform aneurysm clipping after treatment of the infection, with accepting the risk of rebreeding or to perform surgery in an infected environment is yet to be investigated more. Endovascular coiling is another choice for these cases which is not feasible for all patients and requires settings that are not available for us.
Conclusion
In conclusion, VIRs are serious complications of EVD placement in ASH patients. So, first, we have to use a strategy to prevent and decrease the rate of VIR in the patients. Second, if there are simultaneous brain aneurysm and infection induced by external ventricular drainage, the aneurysm clipping is better to be done within 3 days, which also reduces the vasospasm chance or clipping should be postponed after treatment of the infection and done 2-3 weeks later. However, because most of the reviewed studies were retrospective cohort studies, further well-designed and prospective studies are required to verify these results.
Ethical Considerations
Compliance with ethical guidelines
The study protocol was approved by the Ethics Committee of Guilan University of Medical Sciences (Ethical code: IR.GUMS.REC.1397.412). All the study procedures were done in compliance with the ethical guidelines of the Declaration of Helsinki 1957.
Funding
This work was supported by Guilan University of Medical Sciences.
Authors contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
Sakhuja A, Schold JD, Kumar G, Katzan I, Navaneethan SD. Nontraumatic subarachnoid hemorrhage in maintenance dialysis hospitalizations: Trends and outcomes. Stroke. 2014; 45(1):71-6. [DOI:10.1161/STROKEAHA.113.003012] [PMID]
Wu W, Guan Y, Zhao G, Fu XJ, Guo TZ, Liu YT, et al. Elevated IL-6 and TNF-alpha levels in cerebrospinal fluid of subarachnoid hemorrhage patients. Mol Neurobiol. 2016; 53(5):3277-85. [DOI:10.1007/s12035-015-9268-1] [PMID]
Hasan D, Vermeulen M, Wijdicks EF, Hijdra A, Van Gijn J. Management problems in acute hydrocephalus after subarachnoid hemorrhage. Stroke. 1989; 20(6):747-53. [DOI:10.1161/01.STR.20.6.747] [PMID]
Chohan MO, Carlson AP, Hart BL, Yonas H. Lack of functional patency of the lamina terminalis after fenestration following clipping of anterior circulation aneurysms. J Neurosurg. 2013; 119(3):629-33. [DOI:10.3171/2013.5.JNS13251] [PMID]
Germanwala AV, Huang J, Tamargo RJ. Hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am. 2010; 21(2):263-70. [DOI:10.1016/j.nec.2009.10.013] [PMID]
Blount JP, Campbell JA, Haines SJ. Complications in ventricular cerebrospinal fluid shunting. Neurosurg Clin N Am. 1993; 4(4):633-56. [DOI:10.1016/S1042-3680(18)30556-4]
Dasenbrock HH, Rudy RF, Smith TR, Guttieres D, Frerichs KU, Gormley WB, et al. Hospital-acquired infections after aneurysmal subarachnoid hemorrhage: A nationwide analysis. World Neurosurg. 2016; 88:459-74. [DOI:10.1016/j.wneu.2015.10.054] [PMID]
van de Beek D, Drak JM, Tunkel AR. Nosocomial bacterial meningitis. N Engl J Med. 2010; 362(2):146-54. [DOI:10.1056/NEJMra0804573] [PMID]
Hoefnagel D, Dammers R, Ter Laak-Poort MP, Avezaat CJ. Risk factors for infections related to external ventricular drainage. Acta Neurochir. 2008; 150(3):209-14. [DOI:10.1007/s00701-007-1458-9] [PMID]
Lozier AP, Sciacca RR, Romagnoli MF, Connolly Jr ES. Ventriculostomy-related infections: A critical review of the literature. Neurosurg. 2008; 62:688-700. [DOI:10.1227/01.neu.0000316273.35833.7c] [PMID]
Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004; 39(9):1267-84. [DOI:10.1086/425368] [PMID]
Hoogmoed J, van de Beek D, Coert BA, Horn J, Vandertop WP, Verbaan D. Clinical and laboratory characteristics for the diagnosis of bacterial ventriculitis after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2017; 26(3):362-70. [DOI:10.1007/s12028-016-0345-8] [PMID] [PMCID]
Fried HI, Nathan BR, Rowe AS, Zabramski JM, Andaluz N, Bhimraj A, et al. The insertion and management of external ventricular drains: An evidence-based consensus statement: A statement for healthcare professionals from the Neurocritical Care Society. Neurocrit Care. 2016; 24(1):61–81. [DOI:10.1007/s12028-015-0224-8] [PMID]
Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008; 36(5):309-32. [DOI:10.1016/j.ajic.2008.03.002] [PMID]
Walti LN, Conen A, Coward J, Jost GF, Trampuz A. Characteristics of infections associated with external ventricular drains of cerebrospinal fluid. J Infect. 2013; 66(5):424-31. [DOI:10.1016/j.jinf.2012.12.010] [PMID]
Mack WJ, King RG, Ducruet AF, Kreiter K, Mocco J, Maghoub A, et al. Intracranial pressure following aneurysmal subarachnoid hemorrhage: Monitoring practices and outcome data. Neurosurg Focus. 2003; 14(4):1-5. [DOI:10.3171/foc.2003.14.4.3] [PMID]
Mayall CG, Archer NH, Lamb VA, Spadora AC, Baggett JW, Ward JD, et al. Ventriculostomy-related infections; A prospective epidemiologic study. N Engl J Med. 1984; 310(9):553-9. [DOI:10.1056/NEJM198403013100903] [PMID]
Park P, Garton HJ, Kocan MJ, Thompson BG. Thompson BG: Risk of infection with prolonged ventricular catheterization. Neurosurg. 2004; 55(3):594-9. [DOI:10.1227/01.NEU.0000134289.04500.EE] [PMID]
Abla AA, Zabramski JM, Jahnke HK, Fusco D, Nakaji P. Comparison of two antibiotic-impregnated ventricular catheters: A prospective sequential series trial. Neurosurg. 2011; 68(2):437-42. [DOI:10.1227/NEU.0b013e3182039a14] [PMID]
Milhorat TH, Krautheim M. Results of early and delayed operations for ruptured intracranial aneurysms in two series of 100 consecutive patients. Surg Neurol. 1986; 26(2):123-8. [DOI:10.1016/0090-3019(86)90364-2]
Kassell NF, Torner JC. Aneurysmal rebleeding: A preliminary report from the cooperative aneurysm study. Neurosurg. 1983; 13(5):479-81. [DOI:10.1227/00006123-198311000-00001]
Wong GK, Boet R, Ng SC, Chan M, Gin T, Zee B, et al. Ultra-early (within 24 hours) aneurysm treatment after subarachnoid hemorrhage. World Neurosurg. 2012; 77(2):311-15. [DOI:10.1016/j.wneu.2011.09.025] [PMID]
Zentner J, Hoffmann C, Schramm J. Results of early surgery in poor-grade aneurysm patients. J Neurosurg Sci. 1996; 40(3-4):183-8. [PMID]
In this study, we categorized the time period from ictus to aneurysm clipping into three time categories of less than 3 days, 4-14 days, and more than 14 days. In the infection group, 8 patients underwent clipping within 4-14 days and 9 patients after 14 days. In the non-infection group, 13 patients underwent clipping in less than 3 days, and 12 patients in 4-14 days and no patient underwent surgery after 14 days. The mean duration from infection to aneurysm surgical clipping in the infection group was 7.5 days (range: 5-10 days).
Postoperative complications such as morbidity and mortality were evaluated in all patients. Among non-infected patients, 68% of the patients had a good recovery, 4% died and 28% developed severe complications and in non-infected patients who underwent surgery in less 3 days, 84.6% had a good recovery and 7.7% developed severe complications. With regard to the outcome, the best time of clipping is less than 3 days with the least complications (P=0.047).
Among the infected patients, 41.2% of the patients died and 52.9% developed severe complications and 5.9% had a good recovery. In the infected patients who underwent surgery in 4-14 days, 12.5% had a good recovery and 37.5% developed severe complications and 50% of patients died. So the rate of death in patients with surgery in 4-14 days was more than patients with surgery after 2 weeks and the rate of severe complications was more after 2 weeks (Table 2). Multivariate logistic regression analysis showed a significant association between the factors involved in severe complications and postoperative death in patients with VRIs (Table 2).
Discussion
CSF diversion such as EVD is the standard treatment for SAH-induced hydrocephalus. VRIs have been investigated extensively in many studies and infection rates of 0% to 22% and many of risk factors, including SAH, IVH, craniotomy, systemic infection, and longer duration of EVD have been reported [9, 16]. This retrospective analysis found that VRIs rate was 40.4%, which is slightly more than other previous studies. This study indicates that duration of EVD insertion and duration of hospital stay are associated with EVD infection, a finding consistent with several studies in the literature [17-19].
Since the advent of early microsurgical and endovascular approaches to aneurysm treatment, the risk of aneurysm re-rupture has reduced. Timely repair of the ruptured aneurysm by either endovascular coiling or open surgery clipping is a critical element in the effective management of the patients with SAH [20, 21]. Early aneurysm repair in less than 3 days reduces the risk of rebreeding, allows for aggressive management of vasospasm, and relieves restrictions on mobilization and physical therapy, thus resulting in reduced morbidity and mortality compared with the patients receiving delayed surgery [22, 23]. Therefore aneurysm clipping in less 3 days is associated with less morbidity and mortality. Based on our study on the evaluation of non-infected patients, clipping in this time is accompanied by a good outcome. And in the infection group, the aneurysm clipping later than 4 day results in high mortality and morbidity rate.
Among the infected patients, 41.2% died and 52.9% developed severe complications and 5.9% had a good recovery. In the infected patients who underwent surgery between 4 and 14 days, 12.5% had a good recovery and 37.5% developed severe complications and 50% died, with no significant difference with those who underwent surgery after 14 days. Since the in the non-infection group, no patient underwent surgical clipping in less than 3 days and given that the most complications and risk of vasospasm happen between 4 and 14 days, it is better that infected patients undergo surgical clipping after this time or after the recovery of VRIs. However, the time of aneurysm treatment and surgical procedure approach in patients with VRIs have not been determined. This study showed that the placement of a metal clip in the infected environment increases the duration of infection and the length of stay in the hospital and result in poor outcome.
This analysis shows the increased rate of moderate to severe neurological deficits among patients receiving aneurysm clipping during the infection period. So this challenge needs to be further studied. Whether to perform aneurysm clipping after treatment of the infection, with accepting the risk of rebreeding or to perform surgery in an infected environment is yet to be investigated more. Endovascular coiling is another choice for these cases which is not feasible for all patients and requires settings that are not available for us.
Conclusion
In conclusion, VIRs are serious complications of EVD placement in ASH patients. So, first, we have to use a strategy to prevent and decrease the rate of VIR in the patients. Second, if there are simultaneous brain aneurysm and infection induced by external ventricular drainage, the aneurysm clipping is better to be done within 3 days, which also reduces the vasospasm chance or clipping should be postponed after treatment of the infection and done 2-3 weeks later. However, because most of the reviewed studies were retrospective cohort studies, further well-designed and prospective studies are required to verify these results.
Ethical Considerations
Compliance with ethical guidelines
The study protocol was approved by the Ethics Committee of Guilan University of Medical Sciences (Ethical code: IR.GUMS.REC.1397.412). All the study procedures were done in compliance with the ethical guidelines of the Declaration of Helsinki 1957.
Funding
This work was supported by Guilan University of Medical Sciences.
Authors contributions
All authors equally contributed to preparing this article.
Conflict of interest
The authors declared no conflict of interest.
References
Sakhuja A, Schold JD, Kumar G, Katzan I, Navaneethan SD. Nontraumatic subarachnoid hemorrhage in maintenance dialysis hospitalizations: Trends and outcomes. Stroke. 2014; 45(1):71-6. [DOI:10.1161/STROKEAHA.113.003012] [PMID]
Wu W, Guan Y, Zhao G, Fu XJ, Guo TZ, Liu YT, et al. Elevated IL-6 and TNF-alpha levels in cerebrospinal fluid of subarachnoid hemorrhage patients. Mol Neurobiol. 2016; 53(5):3277-85. [DOI:10.1007/s12035-015-9268-1] [PMID]
Hasan D, Vermeulen M, Wijdicks EF, Hijdra A, Van Gijn J. Management problems in acute hydrocephalus after subarachnoid hemorrhage. Stroke. 1989; 20(6):747-53. [DOI:10.1161/01.STR.20.6.747] [PMID]
Chohan MO, Carlson AP, Hart BL, Yonas H. Lack of functional patency of the lamina terminalis after fenestration following clipping of anterior circulation aneurysms. J Neurosurg. 2013; 119(3):629-33. [DOI:10.3171/2013.5.JNS13251] [PMID]
Germanwala AV, Huang J, Tamargo RJ. Hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am. 2010; 21(2):263-70. [DOI:10.1016/j.nec.2009.10.013] [PMID]
Blount JP, Campbell JA, Haines SJ. Complications in ventricular cerebrospinal fluid shunting. Neurosurg Clin N Am. 1993; 4(4):633-56. [DOI:10.1016/S1042-3680(18)30556-4]
Dasenbrock HH, Rudy RF, Smith TR, Guttieres D, Frerichs KU, Gormley WB, et al. Hospital-acquired infections after aneurysmal subarachnoid hemorrhage: A nationwide analysis. World Neurosurg. 2016; 88:459-74. [DOI:10.1016/j.wneu.2015.10.054] [PMID]
van de Beek D, Drak JM, Tunkel AR. Nosocomial bacterial meningitis. N Engl J Med. 2010; 362(2):146-54. [DOI:10.1056/NEJMra0804573] [PMID]
Hoefnagel D, Dammers R, Ter Laak-Poort MP, Avezaat CJ. Risk factors for infections related to external ventricular drainage. Acta Neurochir. 2008; 150(3):209-14. [DOI:10.1007/s00701-007-1458-9] [PMID]
Lozier AP, Sciacca RR, Romagnoli MF, Connolly Jr ES. Ventriculostomy-related infections: A critical review of the literature. Neurosurg. 2008; 62:688-700. [DOI:10.1227/01.neu.0000316273.35833.7c] [PMID]
Tunkel AR, Hartman BJ, Kaplan SL, Kaufman BA, Roos KL, Scheld WM, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004; 39(9):1267-84. [DOI:10.1086/425368] [PMID]
Hoogmoed J, van de Beek D, Coert BA, Horn J, Vandertop WP, Verbaan D. Clinical and laboratory characteristics for the diagnosis of bacterial ventriculitis after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2017; 26(3):362-70. [DOI:10.1007/s12028-016-0345-8] [PMID] [PMCID]
Fried HI, Nathan BR, Rowe AS, Zabramski JM, Andaluz N, Bhimraj A, et al. The insertion and management of external ventricular drains: An evidence-based consensus statement: A statement for healthcare professionals from the Neurocritical Care Society. Neurocrit Care. 2016; 24(1):61–81. [DOI:10.1007/s12028-015-0224-8] [PMID]
Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008; 36(5):309-32. [DOI:10.1016/j.ajic.2008.03.002] [PMID]
Walti LN, Conen A, Coward J, Jost GF, Trampuz A. Characteristics of infections associated with external ventricular drains of cerebrospinal fluid. J Infect. 2013; 66(5):424-31. [DOI:10.1016/j.jinf.2012.12.010] [PMID]
Mack WJ, King RG, Ducruet AF, Kreiter K, Mocco J, Maghoub A, et al. Intracranial pressure following aneurysmal subarachnoid hemorrhage: Monitoring practices and outcome data. Neurosurg Focus. 2003; 14(4):1-5. [DOI:10.3171/foc.2003.14.4.3] [PMID]
Mayall CG, Archer NH, Lamb VA, Spadora AC, Baggett JW, Ward JD, et al. Ventriculostomy-related infections; A prospective epidemiologic study. N Engl J Med. 1984; 310(9):553-9. [DOI:10.1056/NEJM198403013100903] [PMID]
Park P, Garton HJ, Kocan MJ, Thompson BG. Thompson BG: Risk of infection with prolonged ventricular catheterization. Neurosurg. 2004; 55(3):594-9. [DOI:10.1227/01.NEU.0000134289.04500.EE] [PMID]
Abla AA, Zabramski JM, Jahnke HK, Fusco D, Nakaji P. Comparison of two antibiotic-impregnated ventricular catheters: A prospective sequential series trial. Neurosurg. 2011; 68(2):437-42. [DOI:10.1227/NEU.0b013e3182039a14] [PMID]
Milhorat TH, Krautheim M. Results of early and delayed operations for ruptured intracranial aneurysms in two series of 100 consecutive patients. Surg Neurol. 1986; 26(2):123-8. [DOI:10.1016/0090-3019(86)90364-2]
Kassell NF, Torner JC. Aneurysmal rebleeding: A preliminary report from the cooperative aneurysm study. Neurosurg. 1983; 13(5):479-81. [DOI:10.1227/00006123-198311000-00001]
Wong GK, Boet R, Ng SC, Chan M, Gin T, Zee B, et al. Ultra-early (within 24 hours) aneurysm treatment after subarachnoid hemorrhage. World Neurosurg. 2012; 77(2):311-15. [DOI:10.1016/j.wneu.2011.09.025] [PMID]
Zentner J, Hoffmann C, Schramm J. Results of early surgery in poor-grade aneurysm patients. J Neurosurg Sci. 1996; 40(3-4):183-8. [PMID]
Type of Study: Research |
Subject:
Special
Received: 2018/07/5 | Accepted: 2018/11/29 | Published: 2019/01/1
Received: 2018/07/5 | Accepted: 2018/11/29 | Published: 2019/01/1
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |