Thu, Apr 25, 2024
Volume 5, Issue 2 (Spring 2019)
Caspian J Neurol Sci 2019, 5(2): 89-95 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Kashipazha D, Moezzi M, Rafie S, Mehramiri A, Nejati A. The Effect of Intravenous Magnesium Sulfate in Improvement of Acute Ischemic Stroke Induced Disability: A Randomized Double Blinded Clinical Trial. Caspian J Neurol Sci 2019; 5 (2) :89-95
URL: http://cjns.gums.ac.ir/article-1-258-en.html
URL: http://cjns.gums.ac.ir/article-1-258-en.html
1- Department of Neurology, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
2- Department of Emergency Medicine, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
3- Department of Neurology, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran , mehramiri.ac@gmail.com
2- Department of Emergency Medicine, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
3- Department of Neurology, School of Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran , mehramiri.ac@gmail.com
Full-Text [PDF 1162 kb]
(769 Downloads)
| Abstract (HTML) (2398 Views)
Full-Text: (958 Views)
Highlights
● Prescription of magnesium has not impact on outcome of patients with ischemic stroke
Introduction
stroke is one of the most important health problems in the world and the third cause of death in general population [1]. Due to serious stroke-induced disabilities and its socio-economic burden, management in early stages is highly recommended. Currently, recombinant Tissue Plasminogen Activator (r-TPA) is a main treatment of ischemic stroke (80% of cerebrovascular accidents) during the first 3 to 4.5 hours, but it is contraindicated in some patients [2]. Therefore, other adjuvant therapies with neuroprotective agents have been recommended. Magnesium has two main effects: vascular enhancement of flow in cerebral blood vessels and neuronal inhibition of glutamate release during ischemic cascade protection [3, 4]. It is an antagonist of N-Methyl-D-Aspartate (NMDA) receptor and calcium channel which has role in neuronal ischemia-induced death cascade [5, 6]. Cerebral ischemia results in releasing high amounts of glutamate, and then stimulation of NMDA receptor and the final outcome is neuronal death by calcium-dependent signal process (i.e. excitotoxicity) mediated by glutamate. On the other hand blocking the NMDA receptor has many side effect, therefor for maximum safety and efficacy immediately before or after stroke it’s blocking should be targeted by some special drugs [7].
Based on these findings, we aimed to determine the efficacy of magnesium sulfate as a neuroprotective drug. The effect of magnesium upon stroke has been previously investigated, but we intend to evaluate the effect of intravenous magnesium sulfate on improving disabilities induced by acute ischemic stroke.
Materials and Methods
This randomized, double-blind clinical trial (IRCT2015060712781N4) was conducted at Ahvaz Golestan Hospital from 2015 to 2016. We obtained the informed consent from all eligible patients (120 cases) aged 45 to 75, with door-to-needle time of not more than 24 hours for focal persistent neurological symptoms, mNIHSS between 5 to 22, and no bleeding evidence in the initial brain CT scan.
Exclusion criteria were previous or present history of renal failure (serum creatinine level>3mg/dL), heart and respiratory failure (<90% or respiratory rate< 12 breath/min or>24 breath/min), pregnancy, neurodegenerative and neuromuscular disorders, brain tumor, functionally dependent patients, and acute metabolic disorders. Patients were randomly assigned to two groups: the case group received magnesium sulfate and the control group received placebo (normal saline). In addition to the first visit, patients were examined at the end of the first month and 3 months after the administration of drug and the efficiency of treatment was evaluated based on the changes in mNIHSS and mRSS.
The sample size was calculated at the website https://www.stat.ubc.ca/~rollin/stats/ssize/n2.html using an online calculator (56 patients in each group). In this study, the patient and the interviewer were blinded to the applied drug, and all patients were treated routinely.
Patients were admitted to the emergency department, examined by an emergency medicine specialist and were treated by IV saline normal or magnesium sulfate. Then, they were examined by a neurologist who was blinded to the administered medication. All patients received oral antiplatelet agent aspirin 325mg and then 80mg daily. Eligible patients immediately received 4g of IV magnesium sulfate in 200mL saline normal over 20 minutes. Then, 16 g of magnesium sulfate (preparation 50%, Pastor Company) was administered in 800 mL saline normal by infusion pump over 24 hours and repeated for 5 consecutive days.
The placebo group received the same amount of IV normal saline normal. The magnesium level was measured before and after the administration of the drug on the fifth day. The severity of primary disability was recorded on the first day of admission and then at the end of the first month and the third month based on mNIHSS and mRSS in each group.
Quantitative and qualitative data were shown as Mean±SD, counts and percentages, respectively. The independent samples t-test was used to compare the mean of mNIHSS in two groups at each time point. Normal distribution of data was confirmed with Kolmogorov-Smirnov test and qualitative variables were compared with chi-square test. Statistical analysis was performed using SPSS V. 16. P-Value of <0.05 was considered statistically significant
Results
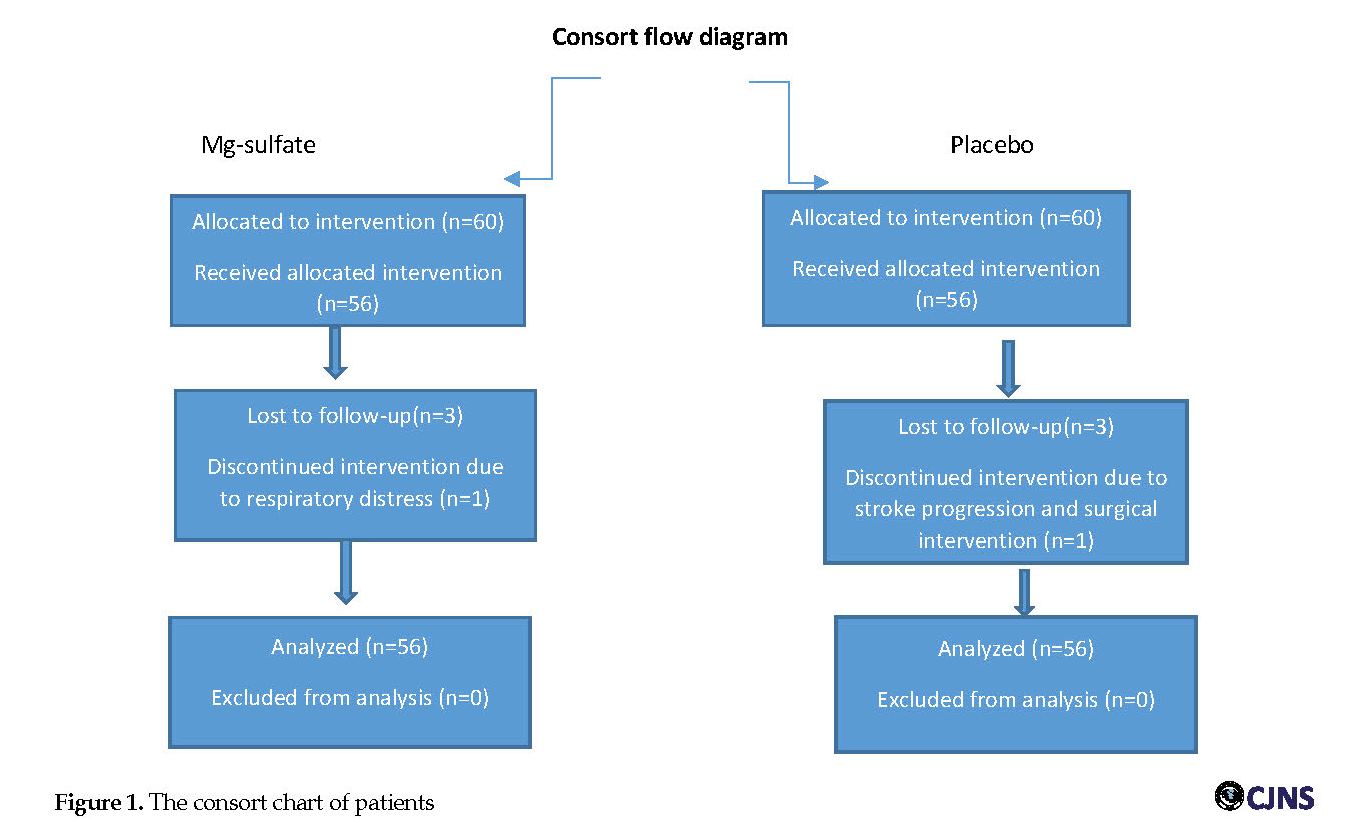
A total of 120 patients with ischemic stroke in middle cerebral artery territory completed the study. Six patients were excluded from the study (Figure 1).
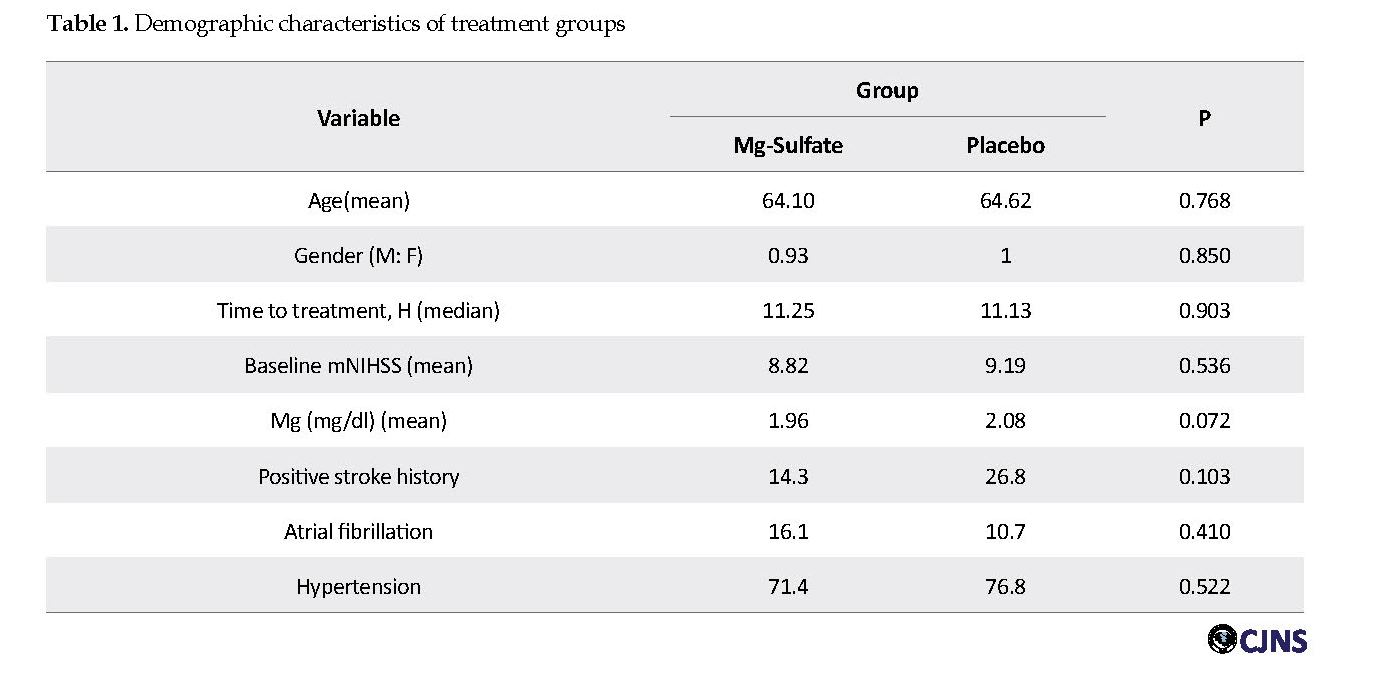
Two patients in the magnesium group, three patients in the control group suffered from headache and one patient developed respiratory distress, which led to their exclusion from the study. Demographic data are shown in Table 1.
● Prescription of magnesium has not impact on outcome of patients with ischemic stroke
Introduction
stroke is one of the most important health problems in the world and the third cause of death in general population [1]. Due to serious stroke-induced disabilities and its socio-economic burden, management in early stages is highly recommended. Currently, recombinant Tissue Plasminogen Activator (r-TPA) is a main treatment of ischemic stroke (80% of cerebrovascular accidents) during the first 3 to 4.5 hours, but it is contraindicated in some patients [2]. Therefore, other adjuvant therapies with neuroprotective agents have been recommended. Magnesium has two main effects: vascular enhancement of flow in cerebral blood vessels and neuronal inhibition of glutamate release during ischemic cascade protection [3, 4]. It is an antagonist of N-Methyl-D-Aspartate (NMDA) receptor and calcium channel which has role in neuronal ischemia-induced death cascade [5, 6]. Cerebral ischemia results in releasing high amounts of glutamate, and then stimulation of NMDA receptor and the final outcome is neuronal death by calcium-dependent signal process (i.e. excitotoxicity) mediated by glutamate. On the other hand blocking the NMDA receptor has many side effect, therefor for maximum safety and efficacy immediately before or after stroke it’s blocking should be targeted by some special drugs [7].
Based on these findings, we aimed to determine the efficacy of magnesium sulfate as a neuroprotective drug. The effect of magnesium upon stroke has been previously investigated, but we intend to evaluate the effect of intravenous magnesium sulfate on improving disabilities induced by acute ischemic stroke.
Materials and Methods
This randomized, double-blind clinical trial (IRCT2015060712781N4) was conducted at Ahvaz Golestan Hospital from 2015 to 2016. We obtained the informed consent from all eligible patients (120 cases) aged 45 to 75, with door-to-needle time of not more than 24 hours for focal persistent neurological symptoms, mNIHSS between 5 to 22, and no bleeding evidence in the initial brain CT scan.
Exclusion criteria were previous or present history of renal failure (serum creatinine level>3mg/dL), heart and respiratory failure (<90% or respiratory rate< 12 breath/min or>24 breath/min), pregnancy, neurodegenerative and neuromuscular disorders, brain tumor, functionally dependent patients, and acute metabolic disorders. Patients were randomly assigned to two groups: the case group received magnesium sulfate and the control group received placebo (normal saline). In addition to the first visit, patients were examined at the end of the first month and 3 months after the administration of drug and the efficiency of treatment was evaluated based on the changes in mNIHSS and mRSS.
The sample size was calculated at the website https://www.stat.ubc.ca/~rollin/stats/ssize/n2.html using an online calculator (56 patients in each group). In this study, the patient and the interviewer were blinded to the applied drug, and all patients were treated routinely.
Patients were admitted to the emergency department, examined by an emergency medicine specialist and were treated by IV saline normal or magnesium sulfate. Then, they were examined by a neurologist who was blinded to the administered medication. All patients received oral antiplatelet agent aspirin 325mg and then 80mg daily. Eligible patients immediately received 4g of IV magnesium sulfate in 200mL saline normal over 20 minutes. Then, 16 g of magnesium sulfate (preparation 50%, Pastor Company) was administered in 800 mL saline normal by infusion pump over 24 hours and repeated for 5 consecutive days.
The placebo group received the same amount of IV normal saline normal. The magnesium level was measured before and after the administration of the drug on the fifth day. The severity of primary disability was recorded on the first day of admission and then at the end of the first month and the third month based on mNIHSS and mRSS in each group.
Quantitative and qualitative data were shown as Mean±SD, counts and percentages, respectively. The independent samples t-test was used to compare the mean of mNIHSS in two groups at each time point. Normal distribution of data was confirmed with Kolmogorov-Smirnov test and qualitative variables were compared with chi-square test. Statistical analysis was performed using SPSS V. 16. P-Value of <0.05 was considered statistically significant
Results
A total of 120 patients with ischemic stroke in middle cerebral artery territory completed the study. Six patients were excluded from the study (Figure 1).
Two patients in the magnesium group, three patients in the control group suffered from headache and one patient developed respiratory distress, which led to their exclusion from the study. Demographic data are shown in Table 1.
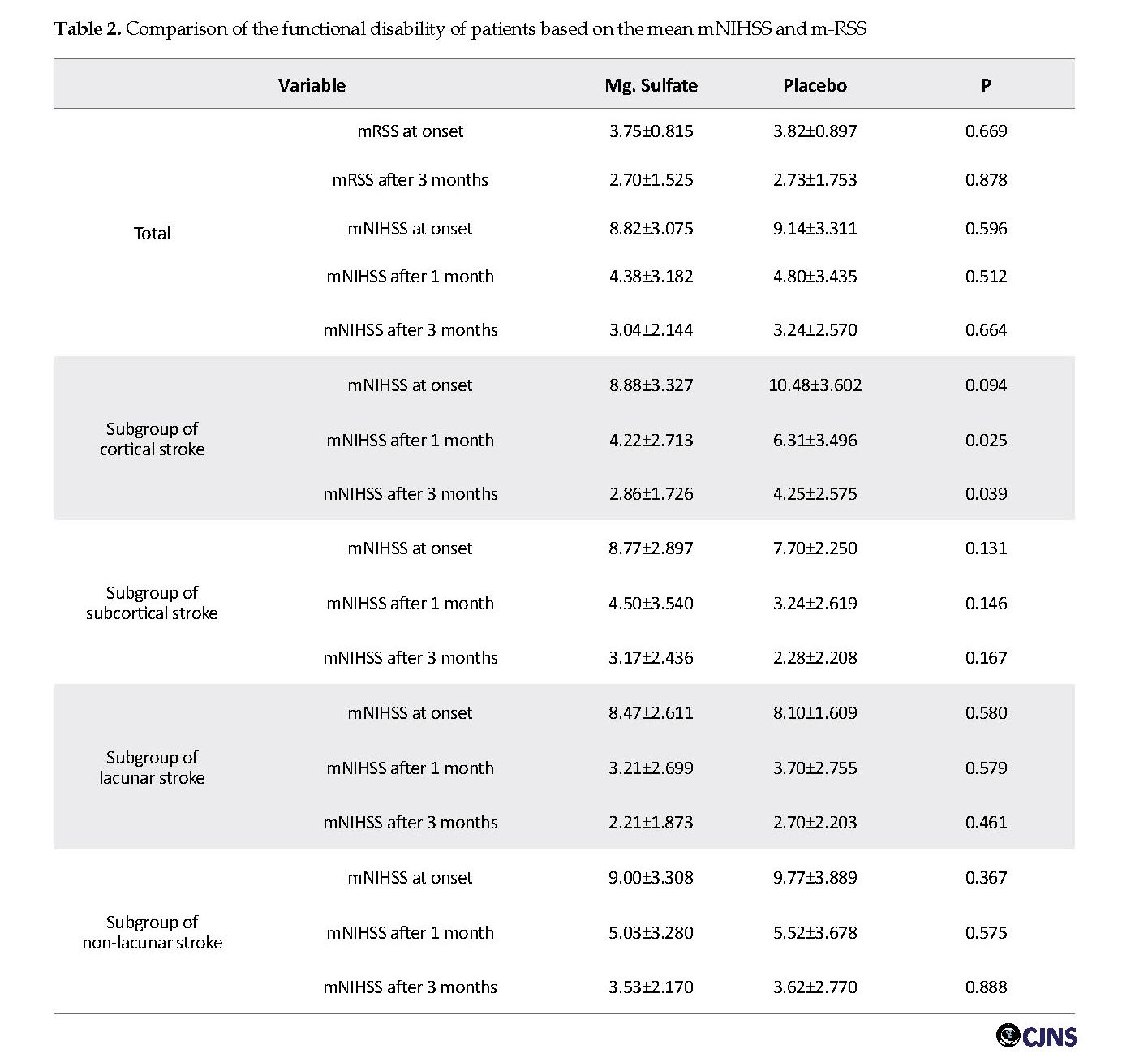
The drug was administrated within 2 to 24 hours from the onset of symptoms, and the mean door-to-needle time was 11.25±5.24 and 11.13±5.61 hours in the case and control groups, respectively. The results indicated that there is no significant difference between the mean of mNIHSS in the two groups. Then, the rate of disability was compared with mNIHSS in both groups over time. The results showed that difference of the mean mNIHSS, one (P=0.512) and three (P=0.664) months after the treatment was not statistically significant between magnesium and placebo groups. Also it was true for mRSS before (P=0.669) and three month (P= 0.878) after the treatment. Based on Table 2, the mean of mNIHSS in patients with cortical stroke after first and third months of treatment was lower in the magnesium sulfate group than that in the placebo group. The difference between the mean of mNIHSS before treatment was not statistically significant, but two groups showed significant differences one and three months after treatment. Therefore, magnesium sulfate significantly improve disability in the cortical stroke cases; but in subcortical stroke subgroups, there was no statistically significant difference between mean of pre- and post-treatment mNIHSS during 1st and 3rd month (p-value 0.131, 0.141 and 0.167, respectively). The patients were classified into two subgroups: lacunar and non-lacunar stroke. In these subgroups also there was not any difference between magnesium and placebo effect after one and three months after stroke. Mortality rate was 8.9% vs. 5.3% in the control group vs. case group (p-value 0.463).
The results showed that difference of the mean mNIHSS, one (P=0.512) and three (P=0.664) months after the treatment was not statistically significant between magnesium and placebo groups. Also it was true for mRSS before (P=0.669) and three month (P= 0.878) after the treatment.
The mean serum magnesium level was 1.96±0.308 mg/dl and 2.08±0.335 mg/dl at baseline in the case and control groups, respectively. At the end of the fifth day, serum magnesium level was 1.94±0.292 mg/dl in the case group and 2.26±0.398 mg/dl in the control group. Despite high serum Mg level in the case group, there was no significant difference observed. Functional disability based on mRSS had not significant difference between two groups in assessment time in this study (Table 2).
Discussion
The present results showed that IV infusion of magnesium sulfate as a neuroprotective agent in patients with acute ischemic stroke in MCA territory did not significantly improved clinical functioning of patients based on mNIHSS criteria after one and three months. In the subgroups of lacunar and non-lacunar infarcts, as well as subcortical subgroups, there was no statistically significant difference between magnesium sulfate group and the placebo group. Only in cortical stroke the mean of mNIHSS showed significant difference between placebo and magnesium groups. In other word the magnesium has impact only on cortical stroke.
Although magnesium therapy in animal models has improved function and healing of tissue ischemia, this fact was not approved in our study in parallel with good patient tolerance and significant increased Mg serum levels at the end of treatment course. Currently, neuroprotection has a remarkable role, but it primarily acts based on the enhancing ischemic tissue tolerance until other defense mechanisms occur.
Calcium has a remarkable role in the degradation and cell death processes by releasing stimulant acids such as glutamate [8]. Many animal studies have supported the beneficial effects of magnesium on the cell body of neurons. Neuroprotection by magnesium probably occurs by both vascular (e.g. blocked voltage gated calcium, released nitric oxide, increased blood flow and inhibition of vasoconstriction) [9] and glial (e.g. blockade of glutaminergic NMDA receptors and inhibition of sodium-calcium ion exchanger) mechanisms [10, 11].
It seems that the magnesium is biologically effective in acute phase of brain infarction [4]. Many animal studies have been conducted on positive neuroprotective process of magnesium. The best neuroprotective dose for magnesium was calculated 2 to 2.5 mmol/L [12]. Some of the neuroprotective drugs which are effective in animal models are not effective in human models. Therefore, early treatment or multi-treatment are suggested [13].
A combined treatment approach of thrombolytic and neuroprotective drugs is a promising strategy in acute ischemic stroke [14]. A safe profile for magnesium sulfate in eclampsia suggest its neuroprotective effect [15]. Some previous studies have clinically shown significant improvement by magnesium sulfate [16-18].
A study in Taiwan evaluated the causes of death among patients with stroke from 1989 to 1993 and reported that higher magnesium levels were associated with a lower incidence of stroke [19]. McKee found that Mg in ischemic area of brain was significantly lower than that in normal control brain [20]. In the IMAGES trial by Meloni, patients received 4 g of magnesium within 15 minutes and 16 g within 24 hours, but there was no significant difference in patients’ functioning based on Barthel’s and Rankin Scale criteria between case and control groups [17].
Mortality rate was 8.9% in placebo group vs. 5.3% in magnesium group without significant difference . But in one study the. Mortality rate after 3 months was higher in the magnesium group than that in the placebo group [21]. The accurate time of assuming neuroprotective effects of drugs is not clear. Saver’s cohort study on 1700 patients with acute stroke in the first 2 hours evaluated the efficacy of magnesium sulfate as a neuroprotective agent in patients before they reached the hospital. No particular benefit was observed after a 90-day follow-up [22]. Brain response to neuroprotective agents may occur within 5 days of stroke [16]. However, neuroprotective agents may cause delayed improvement in healing brain tissue in the later stages of infarction [17]. The extensive, combined and simultaneous evaluations of two or more neuroprotective agents usually recommend administration of drugs at an early stage of infarction.
Conclusion
The present study suggests that IV magnesium sulfate does not have a significant positive effect on the outcome of patients with acute middle cerebral artery infarction. Future studies on a larger population of patients and other adjuvant neuroprotective medications might better show its possible effects. The main limitation of this clinical trial was the sample size. Another multi-center study on a large group of patients will be required to extend the result to other cases.
Ethical Considerations
Compliance with ethical guidelines
The study protocol was approved by the ethics committee of Ahvaz Jundishapur University of Medical Sciences (No. IR.AJUMS.REC.1394.16). All the study procedures were in compliance with the ethical guidelines of the Declaration of Helsinki 1957.
Funding
The fund of this study was provided by the research deputy of Ahvaz Jundishapur University of Medical Sciences
Authors contributions
Davod Kashipazha: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; Meisam Moezzi: Drafting the work or revising it critically for important intellectual content; Shahram Rafie: Substantial contributions to the conception or design of the work; Asieh Mehramiri: Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved, Final approval of the version to be published; Adel Nejati: Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
This research was supported by Ahvaz Jundishapur University of Medical Sciences. We are thankful to our colleagues, Dr.GholamReza Shamsaee, Dr.Seyed Ehsan Mohammadiani Nejad and Dr.Nastaran Majdinasab, Dr.Nilsaz and other colleagues in the department of clinical research development of Golestan Hospital.
References
Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med 2009;7(1):97. [DOI:10.1186/1479-5876-7-97] [PMID] [PMCID]
Matzo M. Early management of ischemic stroke in adults. Am J Nurs 2008;108(8):74. [DOI:10.1097/01.NAJ.0000330274.04045.c4]
Euser AG, Cipolla MJ. Magnesium sulfate for the treatment of eclampsia. Stroke 2009;40(4):1169-75. [DOI:10.1161/STROKEAHA.108.527788] [PMID] [PMCID]
Furukawa Y, Kasai N, Torimitsu K. Effect of Mg 2+ on neural activity of rat cortical and hippocampal neurons in vitro. Magnes Res 2009;22(3):174-81.
Clarke RJ, Johnson JW. Voltage‐dependent gating of NR1/2B NMDA receptors. J Physiol 2008;586(23):5727-41. 2 [DOI:10.1113/jphysiol.2008.160622] [PMID] [PMCID]
Santo-Domingo J, Demaurex N. Calcium uptake mechanisms of mitochondria. Biochim Biophys Acta 2010; 1797(6):907-12. [DOI:10.1016/j.bbabio.2010.01.005] [PMID]
Jovin DG, Katlaps KG, Ellis BK, Dharmaraj B. Neuroprotection against stroke and encephalopathy after cardiac surgery. Interv Med Appl Sci 2019:1-11. [DOI:10.1556/1646.11.2019.01]
Chen H, Luo J, Kintner DB, Shull GE, Sun D. Na+-dependent chloride transporter (NKCC1)-null mice exhibit less gray and white matter damage after focal cerebral ischemia. J Cereb Blood Flow Metab 2005;25(1):54-66. [DOI:10.1038/sj.jcbfm.9600006] [PMID]
Gok B, Okutan O, Beskonakli E, Kilinc K. Effects of magnesium sulphate following spinal cord injury in rats. Chin J Physiol 2007; 50(2):93.
Karadottir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 2005;438:1162-6. [DOI:10.1038/nature04302] [PMID] [PMCID]
Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature 2005;438:1167-71. [DOI:10.1038/nature04301] [PMID]
Westermaier T, Zausinger S, Baethmann A, Schmid-Elsaesser R. Dose finding study of intravenous magnesium sulphate in transient focal cerebral ischemia in rats. Acta Neurochir (Wien) 2005;147(5):525-32. [DOI:10.1007/s00701-005-0496-4] [PMID]
Tymianski M. Novel approaches to neuroprotection trials in acute ischemic stroke. Stroke 2013; 44(10):2942-50. [DOI:10.1161/STROKEAHA.113.000731] [PMID]
Lapchak PA, Araujo DM. Advances in ischemic stroke treatment: neuroprotective and combination therapies. Expert Opin Emerg Drugs 2007;12(1):97-112. [DOI:10.1517/14728214.12.1.97] [PMID]
Ueshima K. Magnesium and ischemic heart disease: a review of epidemiological, experimental, and clinical evidences. Magnes Res 2005;18(4):275-84.
Afshari D, Moradian N, Rezaei M. Evaluation of the intravenous magnesium sulfate effect in clinical improvement of patients with acute ischemic stroke. Clin Neurol Neurosurg 2013;115(4):400-4. [DOI:10.1016/j.clineuro.2012.06.001] [PMID]
Meloni BP, Zhu H, Knuckey NW. Is magnesium neuroprotective following global and focal cerebral ischaemia? A review of published studies. Magnes Res 2006;19(2):123-37.
Majid A. Neuroprotection in stroke: past, present, and future. ISRN Neurol 2014; 515716. [DOI:10.1155/2014/515716] [PMID] [PMCID]
Kaluza J, Orsini N, Levitan EB, Brzozowska A, Roszkowski W, Wolk A. Dietary Calcium and Magnesium Intake and Mortality: A Prospective Study of Men. Am J Epidemiol 2010; 171(7):801-7. [DOI:10.1093/aje/kwp467] [PMID]
McKee JA, Brewer RP, Macy GE, Phillips-Bute B, Campbell KA, Borel CO, et al. Analysis of the brain bioavailability of peripherally administered magnesium sulfate: A study in humans with acute brain injury undergoing prolonged induced hypermagnesemia. Crit Care Med 2005;33(3):661-6. [DOI:10.1097/01.CCM.0000156293.35868.B2] [PMID]
Millán M, Dávalos A. The Need for New Therapies for Acute Ischaemic Stroke. Cerebrovasc Dis 2006;22(1):3-9. [DOI:10.1159/000092327]
Saver JL, Starkman S, Eckstein M, Stratton SJ, Pratt FD, Hamilton S, et al. Prehospital use of magnesium sulfate as neuroprotection in acute stroke. N Engl J Med 2015;5;372(6):528-36. [DOI:10.1056/NEJMoa1408827] [PMID] [PMCID]
The mean serum magnesium level was 1.96±0.308 mg/dl and 2.08±0.335 mg/dl at baseline in the case and control groups, respectively. At the end of the fifth day, serum magnesium level was 1.94±0.292 mg/dl in the case group and 2.26±0.398 mg/dl in the control group. Despite high serum Mg level in the case group, there was no significant difference observed. Functional disability based on mRSS had not significant difference between two groups in assessment time in this study (Table 2).
Discussion
The present results showed that IV infusion of magnesium sulfate as a neuroprotective agent in patients with acute ischemic stroke in MCA territory did not significantly improved clinical functioning of patients based on mNIHSS criteria after one and three months. In the subgroups of lacunar and non-lacunar infarcts, as well as subcortical subgroups, there was no statistically significant difference between magnesium sulfate group and the placebo group. Only in cortical stroke the mean of mNIHSS showed significant difference between placebo and magnesium groups. In other word the magnesium has impact only on cortical stroke.
Although magnesium therapy in animal models has improved function and healing of tissue ischemia, this fact was not approved in our study in parallel with good patient tolerance and significant increased Mg serum levels at the end of treatment course. Currently, neuroprotection has a remarkable role, but it primarily acts based on the enhancing ischemic tissue tolerance until other defense mechanisms occur.
Calcium has a remarkable role in the degradation and cell death processes by releasing stimulant acids such as glutamate [8]. Many animal studies have supported the beneficial effects of magnesium on the cell body of neurons. Neuroprotection by magnesium probably occurs by both vascular (e.g. blocked voltage gated calcium, released nitric oxide, increased blood flow and inhibition of vasoconstriction) [9] and glial (e.g. blockade of glutaminergic NMDA receptors and inhibition of sodium-calcium ion exchanger) mechanisms [10, 11].
It seems that the magnesium is biologically effective in acute phase of brain infarction [4]. Many animal studies have been conducted on positive neuroprotective process of magnesium. The best neuroprotective dose for magnesium was calculated 2 to 2.5 mmol/L [12]. Some of the neuroprotective drugs which are effective in animal models are not effective in human models. Therefore, early treatment or multi-treatment are suggested [13].
A combined treatment approach of thrombolytic and neuroprotective drugs is a promising strategy in acute ischemic stroke [14]. A safe profile for magnesium sulfate in eclampsia suggest its neuroprotective effect [15]. Some previous studies have clinically shown significant improvement by magnesium sulfate [16-18].
A study in Taiwan evaluated the causes of death among patients with stroke from 1989 to 1993 and reported that higher magnesium levels were associated with a lower incidence of stroke [19]. McKee found that Mg in ischemic area of brain was significantly lower than that in normal control brain [20]. In the IMAGES trial by Meloni, patients received 4 g of magnesium within 15 minutes and 16 g within 24 hours, but there was no significant difference in patients’ functioning based on Barthel’s and Rankin Scale criteria between case and control groups [17].
Mortality rate was 8.9% in placebo group vs. 5.3% in magnesium group without significant difference . But in one study the. Mortality rate after 3 months was higher in the magnesium group than that in the placebo group [21]. The accurate time of assuming neuroprotective effects of drugs is not clear. Saver’s cohort study on 1700 patients with acute stroke in the first 2 hours evaluated the efficacy of magnesium sulfate as a neuroprotective agent in patients before they reached the hospital. No particular benefit was observed after a 90-day follow-up [22]. Brain response to neuroprotective agents may occur within 5 days of stroke [16]. However, neuroprotective agents may cause delayed improvement in healing brain tissue in the later stages of infarction [17]. The extensive, combined and simultaneous evaluations of two or more neuroprotective agents usually recommend administration of drugs at an early stage of infarction.
Conclusion
The present study suggests that IV magnesium sulfate does not have a significant positive effect on the outcome of patients with acute middle cerebral artery infarction. Future studies on a larger population of patients and other adjuvant neuroprotective medications might better show its possible effects. The main limitation of this clinical trial was the sample size. Another multi-center study on a large group of patients will be required to extend the result to other cases.
Ethical Considerations
Compliance with ethical guidelines
The study protocol was approved by the ethics committee of Ahvaz Jundishapur University of Medical Sciences (No. IR.AJUMS.REC.1394.16). All the study procedures were in compliance with the ethical guidelines of the Declaration of Helsinki 1957.
Funding
The fund of this study was provided by the research deputy of Ahvaz Jundishapur University of Medical Sciences
Authors contributions
Davod Kashipazha: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; Meisam Moezzi: Drafting the work or revising it critically for important intellectual content; Shahram Rafie: Substantial contributions to the conception or design of the work; Asieh Mehramiri: Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved, Final approval of the version to be published; Adel Nejati: Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgements
This research was supported by Ahvaz Jundishapur University of Medical Sciences. We are thankful to our colleagues, Dr.GholamReza Shamsaee, Dr.Seyed Ehsan Mohammadiani Nejad and Dr.Nastaran Majdinasab, Dr.Nilsaz and other colleagues in the department of clinical research development of Golestan Hospital.
References
Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med 2009;7(1):97. [DOI:10.1186/1479-5876-7-97] [PMID] [PMCID]
Matzo M. Early management of ischemic stroke in adults. Am J Nurs 2008;108(8):74. [DOI:10.1097/01.NAJ.0000330274.04045.c4]
Euser AG, Cipolla MJ. Magnesium sulfate for the treatment of eclampsia. Stroke 2009;40(4):1169-75. [DOI:10.1161/STROKEAHA.108.527788] [PMID] [PMCID]
Furukawa Y, Kasai N, Torimitsu K. Effect of Mg 2+ on neural activity of rat cortical and hippocampal neurons in vitro. Magnes Res 2009;22(3):174-81.
Clarke RJ, Johnson JW. Voltage‐dependent gating of NR1/2B NMDA receptors. J Physiol 2008;586(23):5727-41. 2 [DOI:10.1113/jphysiol.2008.160622] [PMID] [PMCID]
Santo-Domingo J, Demaurex N. Calcium uptake mechanisms of mitochondria. Biochim Biophys Acta 2010; 1797(6):907-12. [DOI:10.1016/j.bbabio.2010.01.005] [PMID]
Jovin DG, Katlaps KG, Ellis BK, Dharmaraj B. Neuroprotection against stroke and encephalopathy after cardiac surgery. Interv Med Appl Sci 2019:1-11. [DOI:10.1556/1646.11.2019.01]
Chen H, Luo J, Kintner DB, Shull GE, Sun D. Na+-dependent chloride transporter (NKCC1)-null mice exhibit less gray and white matter damage after focal cerebral ischemia. J Cereb Blood Flow Metab 2005;25(1):54-66. [DOI:10.1038/sj.jcbfm.9600006] [PMID]
Gok B, Okutan O, Beskonakli E, Kilinc K. Effects of magnesium sulphate following spinal cord injury in rats. Chin J Physiol 2007; 50(2):93.
Karadottir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature 2005;438:1162-6. [DOI:10.1038/nature04302] [PMID] [PMCID]
Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature 2005;438:1167-71. [DOI:10.1038/nature04301] [PMID]
Westermaier T, Zausinger S, Baethmann A, Schmid-Elsaesser R. Dose finding study of intravenous magnesium sulphate in transient focal cerebral ischemia in rats. Acta Neurochir (Wien) 2005;147(5):525-32. [DOI:10.1007/s00701-005-0496-4] [PMID]
Tymianski M. Novel approaches to neuroprotection trials in acute ischemic stroke. Stroke 2013; 44(10):2942-50. [DOI:10.1161/STROKEAHA.113.000731] [PMID]
Lapchak PA, Araujo DM. Advances in ischemic stroke treatment: neuroprotective and combination therapies. Expert Opin Emerg Drugs 2007;12(1):97-112. [DOI:10.1517/14728214.12.1.97] [PMID]
Ueshima K. Magnesium and ischemic heart disease: a review of epidemiological, experimental, and clinical evidences. Magnes Res 2005;18(4):275-84.
Afshari D, Moradian N, Rezaei M. Evaluation of the intravenous magnesium sulfate effect in clinical improvement of patients with acute ischemic stroke. Clin Neurol Neurosurg 2013;115(4):400-4. [DOI:10.1016/j.clineuro.2012.06.001] [PMID]
Meloni BP, Zhu H, Knuckey NW. Is magnesium neuroprotective following global and focal cerebral ischaemia? A review of published studies. Magnes Res 2006;19(2):123-37.
Majid A. Neuroprotection in stroke: past, present, and future. ISRN Neurol 2014; 515716. [DOI:10.1155/2014/515716] [PMID] [PMCID]
Kaluza J, Orsini N, Levitan EB, Brzozowska A, Roszkowski W, Wolk A. Dietary Calcium and Magnesium Intake and Mortality: A Prospective Study of Men. Am J Epidemiol 2010; 171(7):801-7. [DOI:10.1093/aje/kwp467] [PMID]
McKee JA, Brewer RP, Macy GE, Phillips-Bute B, Campbell KA, Borel CO, et al. Analysis of the brain bioavailability of peripherally administered magnesium sulfate: A study in humans with acute brain injury undergoing prolonged induced hypermagnesemia. Crit Care Med 2005;33(3):661-6. [DOI:10.1097/01.CCM.0000156293.35868.B2] [PMID]
Millán M, Dávalos A. The Need for New Therapies for Acute Ischaemic Stroke. Cerebrovasc Dis 2006;22(1):3-9. [DOI:10.1159/000092327]
Saver JL, Starkman S, Eckstein M, Stratton SJ, Pratt FD, Hamilton S, et al. Prehospital use of magnesium sulfate as neuroprotection in acute stroke. N Engl J Med 2015;5;372(6):528-36. [DOI:10.1056/NEJMoa1408827] [PMID] [PMCID]
Type of Study: Research |
Subject:
General
Received: 2019/02/4 | Accepted: 2019/03/15 | Published: 2019/04/1
Received: 2019/02/4 | Accepted: 2019/03/15 | Published: 2019/04/1
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |