Thu, Apr 25, 2024
Volume 4, Issue 3 (Summer 2018)
Caspian J Neurol Sci 2018, 4(3): 114-120 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Fayyazi E, Shaygannejad V, Maljaie M B, Mirmosayyeb O, Badihian S, Moosavian S P. Association Between Sunlight Exposure and Vitamin D Intake and Multiple Sclerosis Disability and Progression. Caspian J Neurol Sci 2018; 4 (3) :114-120
URL: http://cjns.gums.ac.ir/article-1-219-en.html
URL: http://cjns.gums.ac.ir/article-1-219-en.html
Emad Fayyazi1 

 , Vahid Shaygannejad2
, Vahid Shaygannejad2 
 , Mohammad Bagher Maljaie3
, Mohammad Bagher Maljaie3 
 , Omid Mirmosayyeb *
, Omid Mirmosayyeb * 

 4, Shervin Badihian5
4, Shervin Badihian5 
 , Seyedeh Parisa Moosavian5
, Seyedeh Parisa Moosavian5 



 , Vahid Shaygannejad2
, Vahid Shaygannejad2 
 , Mohammad Bagher Maljaie3
, Mohammad Bagher Maljaie3 
 , Omid Mirmosayyeb *
, Omid Mirmosayyeb * 

 4, Shervin Badihian5
4, Shervin Badihian5 
 , Seyedeh Parisa Moosavian5
, Seyedeh Parisa Moosavian5 

1- Neurosciences Research Center, Isfahan University of Medical Sciences, Isfahan, Iran; Department of Neurology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran; Medical Students Research Committee, Isfahan University of Medical Sciences, Isfahan, Iran
2- Neurosciences Research Center, Isfahan University of Medical Sciences, Isfahan, Iran; Department of Neurology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran;
3- Neurosciences Research Center, Isfahan University of Medical Sciences, Isfahan, Iran; Department of Neurology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran; Food Safety Research Center, Isfahan University of Medical Sciences, Isfahan, Iran; Department of Community Nutrition, School of Nutrition and Food Sciences, Isfahan University of Medical Sciences, Isfahan, Iran
4- Neurosciences Research Center, Isfahan University of Medical Sciences, Isfahan, Iran; Department of Neurology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran; Medical Students Research Committee, Isfahan University of Medical Sciences, Isfahan, Iran , Omid.mirmosayyeb@gmail.com
5- Neurosciences Research Center, Isfahan University of Medical Sciences, Isfahan, Iran; Department of Neurology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran; Medical Students Research Committee, Isfahan University of Medical Sciences, Isfahan, Iran
2- Neurosciences Research Center, Isfahan University of Medical Sciences, Isfahan, Iran; Department of Neurology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran;
3- Neurosciences Research Center, Isfahan University of Medical Sciences, Isfahan, Iran; Department of Neurology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran; Food Safety Research Center, Isfahan University of Medical Sciences, Isfahan, Iran; Department of Community Nutrition, School of Nutrition and Food Sciences, Isfahan University of Medical Sciences, Isfahan, Iran
4- Neurosciences Research Center, Isfahan University of Medical Sciences, Isfahan, Iran; Department of Neurology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran; Medical Students Research Committee, Isfahan University of Medical Sciences, Isfahan, Iran , Omid.mirmosayyeb@gmail.com
5- Neurosciences Research Center, Isfahan University of Medical Sciences, Isfahan, Iran; Department of Neurology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran; Medical Students Research Committee, Isfahan University of Medical Sciences, Isfahan, Iran
Full-Text [PDF 1211 kb]
(924 Downloads)
| Abstract (HTML) (2967 Views)
Full-Text: (719 Views)
Introduction
Multiple Sclerosis (MS) is a chronic autoimmune disease of Central Nervous System (CNS) [1], characterized by demyelinated lesions in the brain and spinal cord [2]. It has 3 clinical course types: Relapsing-Remitting MS (RRMS), Primary-Progressive MS (PPMS), and Secondary-Progressive MS (SPMS) [3, 4]. In RRMS, the patient experiences relapses associated with the formation of brain lesions, followed by remissions. In PPMS patients, the CNS neurons degenerate progressively and there are no relapses or remissions [4]. RRMS may progress to SPMS in some cases [4].
Various factors affect MS and its complex course [2]. The pathogenesis of this disease is still not fully recognized; however, a variety of responsible factors are suggested, including environment and nutrition [2, 5, 6]. In addition, MS patients may be at risk for various types of malnutrition or inappropriate diet which worsen their symptoms [6]. Dietary supplements are commonly used among these patients, although the efficacy of these supplements is unclear and requires further investigations [6, 7].
Studies on MS patients have suggested that taking adequate vitamin D can modulate the course of MS and leads to lower incidence of MS [8-10]. However, studies on the relationship between MS severity and MS course and vitamin D intake are limited and reported conflicting findings [11, 12]. Moreover, the main source of vitamin D in the body is sunlight exposure. Vitamin D is suggested to have an inverse association with the progression of MS [8, 13, 14]. Sunlight exposure and vitamin D intake and their relation with MS symptoms is not well investigated by researchers. Here, we aimed to compare sunlight exposure and vitamin D intake between MS phenotypes and evaluate their association with disability and fatigue in each phenotype.
Materials and Methods
Study design and participants
This was a cross-sectional investigation conducted in Isfahan, Iran, in June 2017. A total of 197 MS patients referring to MS Clinic in Kashani Hospital in Isfahan, Iran were recruited. Their brain and spinal cord were assessed by Magnetic Resonance Imaging (MRI) to confirm the diagnosis of MS. We included subjects diagnosed with MS (all types) according to McDonald’s criteria [15]. An equal number of patients from each phenotype of the disease were enrolled in the study. The exclusion criteria consisted of comorbidities or chronic diseases, consuming cholecalciferol, calcium and mineral supplementation, multivitamin or Vitamin D rich foods within 3 months prior to the study, and living in other cities with different latitude.
Procedure and measurements
Demographic data and clinical features including sex, age, weight, height, Expanded Disability Status Scale (EDSS) and systolic and diastolic blood pressure were collected from all study patients. Also, subjects were assessed for sunlight exposure, vitamin D intake, physical activity, and fatigue, as explained in the following paragraphs.
Vitamin D intake assessment
We assessed patients’ dietary intake, especially vitamin D and calcium by a 168-item Food Frequency Questionnaire (FFQ). We reported frequencies of food intakes on a daily, weekly, or monthly basis, over 3 months after enrollment. Calcium content of foods was derived by Nutritionist IV software (First Databank; Hearst; San Bruno; CA; USA). Some Iranian food items were used in the software in accordance with the Food Composition Table of Iran [16]. We evaluated Vitamin D intake considering the “Provisional Table on the Vitamin D Content of Foods” prepared by the United States Department of Agriculture [17].
Sunlight exposure assessment
The questionnaire developed by Glanz et al. was used to measure the mean score of sunlight exposure as well as the amount of time that the patients spend in the sun and lifestyle-related sun exposure habits during the first 3 months after enrollment [18]. It includes questions about the amount of time that patients spent outdoors or in the sun, Sun Protection Factor (SPF), use of sunscreen, skin pigmentation, and clothing type. The total scores of this inventory could range from 4 to 14. Higher scores indicate more sunlight exposure.
Physical activity assessment
We used a self-reported activity questionnaire developed by Aadahl et al. and calculated physical activity by multiplying the obtained score by hour and day. The scores of this questionnaire range from 0.9 (rest/sleep) to 6 (high physical activity). The questionnaire was previously used in studies conducted in Iran [19, 20].
Fatigue Scale assessment
Krupp et al. developed Fatigue Severity Scale (FSS) to assess the severity of fatigue in chronic diseases like MS and lupus [21]. The scores of this scale range from 1 (highly disagree) to 7 (highly agree/high fatigue levels). FSS evaluates fatigue in 9 items based on a Likert scale. The Persian version of this scale obtained the internal consistency (Cronbach’s alpha) and test-retest reliability as 0.98 and 0.93, respectively.
Statistical analysis
Normal distribution of variables among SPMS, RRMS and PPMS participants was examined by Shapiro-Wilk test. All variables including EDSS, fatigue scale score, vitamin D intake and sunlight exposure scores were normally distributed. We used 1-way Analysis of Variance (ANOVA) for comparing the mean scores of variables between MS subgroups. Sex and age were considered as covariates in 1-way ANOVA; however, they did not affect the results and therefore were excluded from the analysis. We used Pearson correlation test to assess the correlation between MS subtypes and fatigue scale, EDSS, vitamin D intake and sunlight exposure. SPSS was used for statistical analysis. P values of <0.05 were set as significant.
Results
A total of 197 MS patients were initially included and 71 of them were excluded because of comorbidities. The final study population included 42 patients of SPMS, RRMS, and PPMS. Demographic characteristics of cases are presented in Table 1. According to the presented data, age, gender, diastolic and systolic blood pressure did not have any statistical differences between SPMS, RRMS and PPMS groups, while BMI and weight were statistically different between the groups.
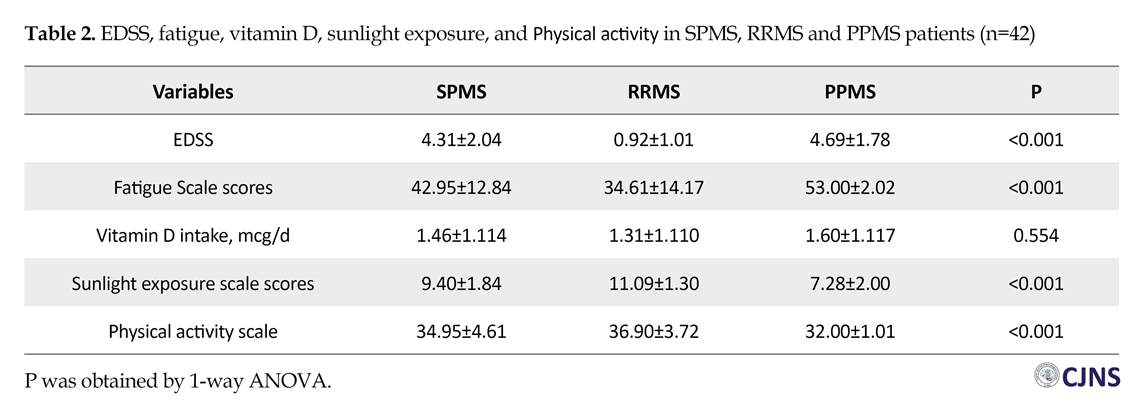
The Mean±SD scores of fatigue scale, EDSS, vitamin D intake and sunlight exposure are listed in Table 2. According to the results, EDSS and the scores of fatigue scale in the PPMS (4.69±1.78 and 53.00±2.02) and SPMS (4.31±2.04 and 42.95±12.84) groups were higher than the RRMS group (0.92±1.01 and 53.00±2.02). In addition, patients in the RRMS group had significantly higher scores of sunlight exposure (11.09±1.30) and physical activity (36.90±3.72), compared to the SPMS (9.40±1.84 and 34.95±4.61) and PPMS (7.28±2.00 and 32.00±1.01) groups. Vitamin D intake was not statistically different between the groups.
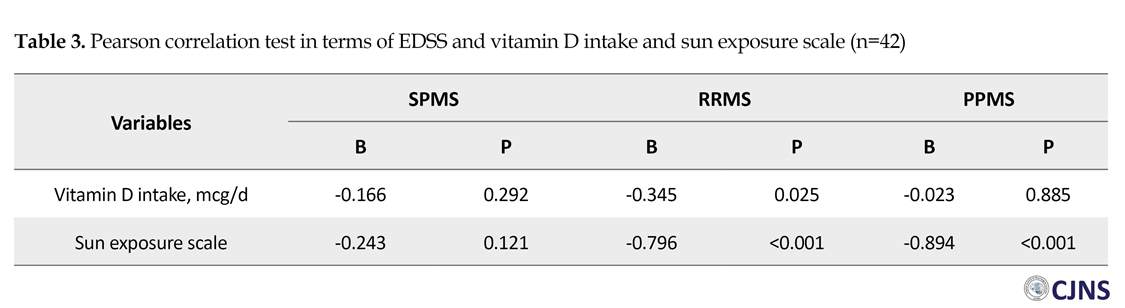
Pearson correlation test results of EDSS with respect to the scores of vitamin D intake and sunlight exposure in each group are presented in Table 3. We found a negative relationship between vitamin D intake and EDSS in the RRMS group (r=-0.345, P=0.025). Also, a negative correlation was found between sunlight exposure scores and EDSS in the RRMS (r=-0.796, P<0.001) and the PPMS (r=-0.894, P<0.001) groups. Pearson correlation test for the score of fatigue scale with respect to vita
Multiple Sclerosis (MS) is a chronic autoimmune disease of Central Nervous System (CNS) [1], characterized by demyelinated lesions in the brain and spinal cord [2]. It has 3 clinical course types: Relapsing-Remitting MS (RRMS), Primary-Progressive MS (PPMS), and Secondary-Progressive MS (SPMS) [3, 4]. In RRMS, the patient experiences relapses associated with the formation of brain lesions, followed by remissions. In PPMS patients, the CNS neurons degenerate progressively and there are no relapses or remissions [4]. RRMS may progress to SPMS in some cases [4].
Various factors affect MS and its complex course [2]. The pathogenesis of this disease is still not fully recognized; however, a variety of responsible factors are suggested, including environment and nutrition [2, 5, 6]. In addition, MS patients may be at risk for various types of malnutrition or inappropriate diet which worsen their symptoms [6]. Dietary supplements are commonly used among these patients, although the efficacy of these supplements is unclear and requires further investigations [6, 7].
Studies on MS patients have suggested that taking adequate vitamin D can modulate the course of MS and leads to lower incidence of MS [8-10]. However, studies on the relationship between MS severity and MS course and vitamin D intake are limited and reported conflicting findings [11, 12]. Moreover, the main source of vitamin D in the body is sunlight exposure. Vitamin D is suggested to have an inverse association with the progression of MS [8, 13, 14]. Sunlight exposure and vitamin D intake and their relation with MS symptoms is not well investigated by researchers. Here, we aimed to compare sunlight exposure and vitamin D intake between MS phenotypes and evaluate their association with disability and fatigue in each phenotype.
Materials and Methods
Study design and participants
This was a cross-sectional investigation conducted in Isfahan, Iran, in June 2017. A total of 197 MS patients referring to MS Clinic in Kashani Hospital in Isfahan, Iran were recruited. Their brain and spinal cord were assessed by Magnetic Resonance Imaging (MRI) to confirm the diagnosis of MS. We included subjects diagnosed with MS (all types) according to McDonald’s criteria [15]. An equal number of patients from each phenotype of the disease were enrolled in the study. The exclusion criteria consisted of comorbidities or chronic diseases, consuming cholecalciferol, calcium and mineral supplementation, multivitamin or Vitamin D rich foods within 3 months prior to the study, and living in other cities with different latitude.
Procedure and measurements
Demographic data and clinical features including sex, age, weight, height, Expanded Disability Status Scale (EDSS) and systolic and diastolic blood pressure were collected from all study patients. Also, subjects were assessed for sunlight exposure, vitamin D intake, physical activity, and fatigue, as explained in the following paragraphs.
Vitamin D intake assessment
We assessed patients’ dietary intake, especially vitamin D and calcium by a 168-item Food Frequency Questionnaire (FFQ). We reported frequencies of food intakes on a daily, weekly, or monthly basis, over 3 months after enrollment. Calcium content of foods was derived by Nutritionist IV software (First Databank; Hearst; San Bruno; CA; USA). Some Iranian food items were used in the software in accordance with the Food Composition Table of Iran [16]. We evaluated Vitamin D intake considering the “Provisional Table on the Vitamin D Content of Foods” prepared by the United States Department of Agriculture [17].
Sunlight exposure assessment
The questionnaire developed by Glanz et al. was used to measure the mean score of sunlight exposure as well as the amount of time that the patients spend in the sun and lifestyle-related sun exposure habits during the first 3 months after enrollment [18]. It includes questions about the amount of time that patients spent outdoors or in the sun, Sun Protection Factor (SPF), use of sunscreen, skin pigmentation, and clothing type. The total scores of this inventory could range from 4 to 14. Higher scores indicate more sunlight exposure.
Physical activity assessment
We used a self-reported activity questionnaire developed by Aadahl et al. and calculated physical activity by multiplying the obtained score by hour and day. The scores of this questionnaire range from 0.9 (rest/sleep) to 6 (high physical activity). The questionnaire was previously used in studies conducted in Iran [19, 20].
Fatigue Scale assessment
Krupp et al. developed Fatigue Severity Scale (FSS) to assess the severity of fatigue in chronic diseases like MS and lupus [21]. The scores of this scale range from 1 (highly disagree) to 7 (highly agree/high fatigue levels). FSS evaluates fatigue in 9 items based on a Likert scale. The Persian version of this scale obtained the internal consistency (Cronbach’s alpha) and test-retest reliability as 0.98 and 0.93, respectively.
Statistical analysis
Normal distribution of variables among SPMS, RRMS and PPMS participants was examined by Shapiro-Wilk test. All variables including EDSS, fatigue scale score, vitamin D intake and sunlight exposure scores were normally distributed. We used 1-way Analysis of Variance (ANOVA) for comparing the mean scores of variables between MS subgroups. Sex and age were considered as covariates in 1-way ANOVA; however, they did not affect the results and therefore were excluded from the analysis. We used Pearson correlation test to assess the correlation between MS subtypes and fatigue scale, EDSS, vitamin D intake and sunlight exposure. SPSS was used for statistical analysis. P values of <0.05 were set as significant.
Results
A total of 197 MS patients were initially included and 71 of them were excluded because of comorbidities. The final study population included 42 patients of SPMS, RRMS, and PPMS. Demographic characteristics of cases are presented in Table 1. According to the presented data, age, gender, diastolic and systolic blood pressure did not have any statistical differences between SPMS, RRMS and PPMS groups, while BMI and weight were statistically different between the groups.
The Mean±SD scores of fatigue scale, EDSS, vitamin D intake and sunlight exposure are listed in Table 2. According to the results, EDSS and the scores of fatigue scale in the PPMS (4.69±1.78 and 53.00±2.02) and SPMS (4.31±2.04 and 42.95±12.84) groups were higher than the RRMS group (0.92±1.01 and 53.00±2.02). In addition, patients in the RRMS group had significantly higher scores of sunlight exposure (11.09±1.30) and physical activity (36.90±3.72), compared to the SPMS (9.40±1.84 and 34.95±4.61) and PPMS (7.28±2.00 and 32.00±1.01) groups. Vitamin D intake was not statistically different between the groups.
Pearson correlation test results of EDSS with respect to the scores of vitamin D intake and sunlight exposure in each group are presented in Table 3. We found a negative relationship between vitamin D intake and EDSS in the RRMS group (r=-0.345, P=0.025). Also, a negative correlation was found between sunlight exposure scores and EDSS in the RRMS (r=-0.796, P<0.001) and the PPMS (r=-0.894, P<0.001) groups. Pearson correlation test for the score of fatigue scale with respect to vita
min D intake and sunlight exposure scores in each group are listed in Table 4. There was a negative correlation between fatigue scores and sunlight exposure scores in the RRMS (r=-0.402, P=0.008) and the PPMS (r=0.650, P<0.001) groups.
Discussion
MS phenotypes are clinically defined based on the different disease courses. The most common type of MS is RRMS, marked by relapses followed by periods of remission where symptoms improve or may disappear [4, 22]. In SPMS, patients experience continued worsening or stability of symptoms and in PPMS, symptoms worsen gradually [4, 22]. According to these differences, RRMS patients are expected to experience less disabilities and perform more physical activity. This explains our findings on lower EDSS and higher physical activity scale in the RRMS group, as well as greater weight and BMI in the PPMS group, due to less physical activity. Fatigue is the most frequently reported symptom by MS patients occurring in 50%-90% of cases [23, 24].
The present study showed higher scores of fatigue scale in PPMS group. This can be explained by the fact that the severity of fatigue is correlated with the disease severity and activity [24]. One of the environmental factors that affects the risk of developing MS is vitamin D [8, 25]. Inadequate vitamin D intake is correlated with a higher risk for MS [9, 26]. Also, higher vitamin D intake in dietary regimen leads to lower incidence of MS [10, 27]. On the other hand, increased risk of relapses is related to low serum levels of vitamin D [28, 29].
Simson et al. showed that a 10 nmol/L increase of serum levels of 25 (OH)-vitamin D reduces the risk for relapse up to 9%-12% in RRMS patients [30]. Moreover, it is shown that vitamin D serum levels are inversely associated with disease severity [31-33]. These findings confirm the immunomodulatory effect of vitamin D in MS. The serum levels of vitamin D were not evaluated in this study; however, the effect of vitamin D intake was not significantly different between the MS types.
Discussion
MS phenotypes are clinically defined based on the different disease courses. The most common type of MS is RRMS, marked by relapses followed by periods of remission where symptoms improve or may disappear [4, 22]. In SPMS, patients experience continued worsening or stability of symptoms and in PPMS, symptoms worsen gradually [4, 22]. According to these differences, RRMS patients are expected to experience less disabilities and perform more physical activity. This explains our findings on lower EDSS and higher physical activity scale in the RRMS group, as well as greater weight and BMI in the PPMS group, due to less physical activity. Fatigue is the most frequently reported symptom by MS patients occurring in 50%-90% of cases [23, 24].
The present study showed higher scores of fatigue scale in PPMS group. This can be explained by the fact that the severity of fatigue is correlated with the disease severity and activity [24]. One of the environmental factors that affects the risk of developing MS is vitamin D [8, 25]. Inadequate vitamin D intake is correlated with a higher risk for MS [9, 26]. Also, higher vitamin D intake in dietary regimen leads to lower incidence of MS [10, 27]. On the other hand, increased risk of relapses is related to low serum levels of vitamin D [28, 29].
Simson et al. showed that a 10 nmol/L increase of serum levels of 25 (OH)-vitamin D reduces the risk for relapse up to 9%-12% in RRMS patients [30]. Moreover, it is shown that vitamin D serum levels are inversely associated with disease severity [31-33]. These findings confirm the immunomodulatory effect of vitamin D in MS. The serum levels of vitamin D were not evaluated in this study; however, the effect of vitamin D intake was not significantly different between the MS types.
According to cross-sectional studies, higher vitamin D level is also correlated with lower scores of EDSS in MS patients [32-34]. Additionally, Ascherio et al. measured the serum levels of 25 (OH)-vitamin D in a prospective study for 5 years, looking for brain lesions on MRI as well as relapses and EDSS. They found that vitamin D can affect disease progression and activity, because there was an inverse relationship between the serum levels of vitamin D and the disease severity [35]. This study found that higher vitamin D intake is inversely correlated with the scores of EDSS in RRMS patients, which is consistent with previous findings.
There is a correlation between sunlight exposure, skin color, use of sunscreen, sun avoidance behaviors, the geographical features of the region and the season [9]. It is strongly related to the serum levels of 25 (OH)-vitamin D and have immunosuppressive effects, both independently and due to vitamin D production [9, 36]. Investigations on the association of sunlight exposure and MS progression have reported inconsistent findings, mostly because of different measurement approaches and study designs. A case-control investigation revealed that a considerable number of patients have spent at least 2 hours outdoors per day in the summer during childhood, while another case-control study reported no relationship between the time spent outdoors in the summer and disease progression [37, 38].
In both studies, results were affected by selection bias. In contrast, recent studies have provided evidence on the protective effect of sunlight exposure for MS progression [39]. A study reported that MS risk is inversely correlated with sunlight exposure, using actinic skin damage for measuring sun exposure [13]. Another study reported similar findings using sunlight exposure questionnaires 12:53 PM. Similarly, a study on monozygotic twins showed lower sunlight exposure in MS cases, compared to their healthy twin [40].
Despite these findings, limited studies have evaluated the effect of sunlight exposure on MS progression and severity. Tremlett et al. prospectively followed a group of RRMS patients in southern Tasmania and evaluated the association between some environmental factors and MS relapse. They reported an inverse association between ultraviolet radiation and relapse rates after an average follow up of 2.3 years [41]. MS mortality is also seen to be lower in outdoor workers in areas with high sunlight intensity [42].
In this study, patients in RRMS and PPMS groups reported more fatigue when they were exposed to sunlight for fewer hours. Although none of the previous studies have evaluated the association between sunlight exposure, disability and fatigue, our results seem to be consistent with the theory of protective and immunomodulatory effect of sunlight exposure and ultraviolet radiation in MS [9].
Conclusion
This study evaluated the effect of adequate vitamin D intake and sunlight exposure on MS severity and course. In the RRMS patients, vitamin D intake was inversely correlated with the scores of EDSS. Also, we found that sunlight exposure is inversely associated with the scores of EDSS and fatigue in PPMS and RRMS patients. Further studies with stronger methodologies and larger sample sizes are required to evaluate our findings.
One limitation to our study was that participants were not selected randomly and were not properly matched which may lead to selection bias. Also, the obtained associations may be interpreted inversely, due to the reverse causation bias that may have happened because of the study design. Eventually, sunlight exposure was measured using a questionnaire, while more precise methods like actinic damage may result in different findings. Despite these limitation, we tried to minimize the recall bias by conducting the study, prospectively. Also, we tried to include all MS types, while previous studies mostly focused on RRMS.
Ethical Considerations
Compliance with ethical guidelines
An informed consent was taken from all participants before enrollment. The study was approved by the research ethics committee of Isfahan University of Medical Sciences (Ethical Code No: IR.MUI.REC.1394.1.329).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
The authors certify that they have no affiliation with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials dismissed in this manuscript.
Acknowledgements
The authors wish to thank the patients for their cooperation throughout the follow-up process.
References
There is a correlation between sunlight exposure, skin color, use of sunscreen, sun avoidance behaviors, the geographical features of the region and the season [9]. It is strongly related to the serum levels of 25 (OH)-vitamin D and have immunosuppressive effects, both independently and due to vitamin D production [9, 36]. Investigations on the association of sunlight exposure and MS progression have reported inconsistent findings, mostly because of different measurement approaches and study designs. A case-control investigation revealed that a considerable number of patients have spent at least 2 hours outdoors per day in the summer during childhood, while another case-control study reported no relationship between the time spent outdoors in the summer and disease progression [37, 38].
In both studies, results were affected by selection bias. In contrast, recent studies have provided evidence on the protective effect of sunlight exposure for MS progression [39]. A study reported that MS risk is inversely correlated with sunlight exposure, using actinic skin damage for measuring sun exposure [13]. Another study reported similar findings using sunlight exposure questionnaires 12:53 PM. Similarly, a study on monozygotic twins showed lower sunlight exposure in MS cases, compared to their healthy twin [40].
Despite these findings, limited studies have evaluated the effect of sunlight exposure on MS progression and severity. Tremlett et al. prospectively followed a group of RRMS patients in southern Tasmania and evaluated the association between some environmental factors and MS relapse. They reported an inverse association between ultraviolet radiation and relapse rates after an average follow up of 2.3 years [41]. MS mortality is also seen to be lower in outdoor workers in areas with high sunlight intensity [42].
In this study, patients in RRMS and PPMS groups reported more fatigue when they were exposed to sunlight for fewer hours. Although none of the previous studies have evaluated the association between sunlight exposure, disability and fatigue, our results seem to be consistent with the theory of protective and immunomodulatory effect of sunlight exposure and ultraviolet radiation in MS [9].
Conclusion
This study evaluated the effect of adequate vitamin D intake and sunlight exposure on MS severity and course. In the RRMS patients, vitamin D intake was inversely correlated with the scores of EDSS. Also, we found that sunlight exposure is inversely associated with the scores of EDSS and fatigue in PPMS and RRMS patients. Further studies with stronger methodologies and larger sample sizes are required to evaluate our findings.
One limitation to our study was that participants were not selected randomly and were not properly matched which may lead to selection bias. Also, the obtained associations may be interpreted inversely, due to the reverse causation bias that may have happened because of the study design. Eventually, sunlight exposure was measured using a questionnaire, while more precise methods like actinic damage may result in different findings. Despite these limitation, we tried to minimize the recall bias by conducting the study, prospectively. Also, we tried to include all MS types, while previous studies mostly focused on RRMS.
Ethical Considerations
Compliance with ethical guidelines
An informed consent was taken from all participants before enrollment. The study was approved by the research ethics committee of Isfahan University of Medical Sciences (Ethical Code No: IR.MUI.REC.1394.1.329).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
The authors certify that they have no affiliation with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials dismissed in this manuscript.
Acknowledgements
The authors wish to thank the patients for their cooperation throughout the follow-up process.
References
- Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1, 25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007; 179(3):1634-47. [DOI:10.4049/jimmunol.179.3.1634] [PMID]
- Ciccarelli O, Thompson A. Multiple Sclerosis in 2015: Managing the complexity of Multiple Sclerosis. Nat Rev Neurol. 2016; 12(2):70-2. [DOI:10.1038/nrneurol.2016.2] [PMID]
- Dutta R, Trapp BD. Relapsing and progressive forms of Multiple Sclerosis–insights from pathology. Curr Opin Neurol. 2014; 27(3):271-8. [DOI:10.1097/WCO.0000000000000094] [PMID] [PMCID]
- Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sørensen PS, Thompson AJ, et al. Defining the clinical course of Multiple Sclerosis The 2013 revisions. Neurol. 2014; 83(3):278-86. [DOI:10.1212/WNL.0000000000000560] [PMID] [PMCID]
- Leray E, Moreau T, Fromont A, Edan G. Epidemiology of Multiple Sclerosis. Rev Neurol. 2016; 172(1):3-13. [DOI:10.1016/j.neurol.2015.10.006] [PMID]
- Riccio P, Rossano R. Nutrition facts in Multiple Sclerosis. ASN Neuro. 2015; 7(1):1759091414568185. [DOI:10.1177/1759091414568185] [PMID] [PMCID]
- Riccio P. The molecular basis of nutritional intervention in Multiple Sclerosis: a narrative review. Complement Ther Med. 2011; 19(4):228-37. [DOI:10.1016/j.ctim.2011.06.006] [PMID]
- Ascherio A, Munger KL. Environmental risk factors for Multiple Sclerosis. Part II: Noninfectious factors. Ann Neurol. 2007; 61(6):504-13. [DOI:10.1002/ana.21141] [PMID]
- Ascherio A, Munger KL, Simon KC. Vitamin D and Multiple Sclerosis. Lancet Neurol. 2010; 9(6):599-612. [DOI:10.1016/S1474-4422(10)70086-7]
- Munger KL, Zhang S, O’reilly E, Hernan M, Olek M, Willett W, et al. Vitamin D intake and incidence of Multiple Sclerosis. Neurol. 2004; 62(1):60-5. [DOI:10.1212/01.WNL.0000101723.79681.38] [PMID]
- Muris AH, Smolders J, Rolf L, Klinkenberg LJ, van der Linden N, Meex S, et al. Vitamin D status does not affect disability progression of patients with Multiple Sclerosis over three year follow-up. PloS One. 2016; 11(6):e0156122. [DOI:10.1371/journal.pone.0156122] [PMID] [PMCID]
- Esmael A, El-Sherif M, Elazzouny AA. Effects of vitamin D deficiency on the relapse, severity, and disability of Multiple Sclerosis. Egypt J Neurol Psychiatr Neurosurg. 2016; 53(3):174-8. [DOI:10.4103/1110-1083.193086]
- van der Mei IA, Ponsonby AL, Dwyer T, Blizzard L, Simmons R, Taylor B, et al. Past exposure to sun, skin phenotype, and risk of Multiple Sclerosis: Case-control study. Bmj. 2003; 327(7410):316. [DOI:10.1136/bmj.327.7410.316] [PMID] [PMCID]
- Langer-Gould A, Chen L, Gonzales E, Schroeder C, Xiang A, Barcellos L, et al. Lifetime Sun Exposure and the Risk of Multiple Sclerosis in Blacks and Hispanics (P3. 002). Neurol. 2016; 86(16 Supplement).
- Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for Multiple Sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011; 69(2):292-302. [DOI:10.1002/ana.22366] [PMID] [PMCID]
- Azar M, Sarkisian E. Food composition table of Iran: National Nutrition and Food Research Institute. Tehran: Shahid Beheshti University; 1980.
- Weihrauch J, Neira P. Provisional table on the vitamin D content of foods. Washington DC: US Department of Agriculture; 1991.
- Glanz K, Yaroch AL, Dancel M, Saraiya M, Crane LA, Buller DB, et al. Measures of sun exposure and sun protection practices for behavioral and epidemiologic research. Arch Dermatol. 2008; 144(2):217-22. [DOI:10.1001/archdermatol.2007.46] [PMID]
- Aadahl M, JØrgensen T. Validation of a new self-report instrument for measuring physical activity. Med Sci Sports Exerc. 2003; 35(7):1196-202. [DOI:10.1249/01.MSS.0000074446.02192.14] [PMID]
- Rezazadeh A, Rashidkhani B, Omidvar N. Association of major dietary patterns with socioeconomic and lifestyle factors of adult women living in Tehran, Iran. Nutr. 2010; 26(3):337-41. [DOI:10.1016/j.nut.2009.06.019] [PMID]
- Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale: Application to patients with Multiple Sclerosis and systemic lupus erythematosus. Arch Neurol. 1989; 46(10):1121-3. [DOI:10.1001/archneur.1989.00520460115022] [PMID]
- Goldenberg MM. Multiple Sclerosis review. Phys Ther. 2012; 37(3):175-84. [PMID] [PMCID]
- Braley TJ, Chervin RD. Fatigue in Multiple Sclerosis: Mechanisms, evaluation, and treatment. Sleep. 2010; 33(8):1061. [DOI:10.1093/sleep/33.8.1061]
- Rottoli M, La Gioia S, Frigeni B, Barcella V. Pathophysiology, assessment and management of Multiple Sclerosis fatigue: An update. Expert Rev Neurother. 2017; 17(4):373-9. [DOI:10.1080/14737175.2017.1247695.] [PMID]
- Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of Multiple Sclerosis. JAMA. 2006; 296(23):2832-8. [DOI:10.1001/jama.296.23.2832] [PMID]
- Salzer J, Hallmans G, Nyström M, Stenlund H, Wadell G, Sundström P. Vitamin D as a protective factor in Multiple Sclerosis. Neurol. 2012; 79(21):2140-5. [DOI:10.1212/WNL.0b013e3182752ea8] [PMID]
- Kampman M, Wilsgaard T, Mellgren S. Outdoor activities and diet in childhood and adolescence relate to MS risk above the Arctic Circle. J Neurol. 2007; 254(4):471-7. [DOI:10.1007/s00415-006-0395-5] [PMID]
- Mowry EM, Krupp LB, Milazzo M, Chabas D, Strober JB, Belman AL, et al. Vitamin D status is associated with relapse rate in pediatric‐onset Multiple Sclerosis. Ann Neurol. 2010; 67(5):618-24. [PMID]
- Correale J, Ysrraelit MC, Gaitán MI. Immunomodulatory effects of Vitamin D in Multiple Sclerosis. Brain. 2009; 132(5):1146-60. [DOI:10.1093/brain/awp033] [PMID]
- Simpson S, Taylor B, Blizzard L, Ponsonby AL, Pittas F, Tremlett H, et al. Higher 25‐hydroxyvitamin D is associated with lower relapse risk in Multiple Sclerosis. Ann Neurol. 2010; 68(2):193-203. [PMID]
- Soilu-Hänninen M, Airas L, Mononen I, Heikkilä A, Viljanen M, Hänninen A. 25-Hydroxyvitamin D levels in serum at the onset of Multiple Sclerosis. Mult Scler. 2005; 11(3):266-71. [DOI:10.1191/1352458505ms1157oa] [PMID]
- Smolders J, Menheere P, Kessels A, Damoiseaux J, Hupperts R. Association of vitamin D metabolite levels with relapse rate and disability in Multiple Sclerosis. Mult Scler. 2008; 14(9):1220-4. [DOI:10.1177/1352458508094399] [PMID]
- van der Mei IA, Ponsonby A-L, Dwyer T, Blizzard L, Taylor BV, Kilpatrick T, et al. Vitamin D levels in people with Multiple Sclerosis and community controls in Tasmania, Australia. J Neurol. 2007; 254(5):581. [DOI:10.1007/s00415-006-0315-8] [PMID]
- Weinstock-Guttman B, Zivadinov R, Qu J, Cookfair D, Duan X, Bang E, et al. Vitamin D metabolites are associated with clinical and MRI outcomes in Multiple Sclerosis patients. J Neurol, Neurosurg & Psychiatry. 2011; 82(2):189-95. [DOI:10.1136/jnnp.2010.227942] [PMID]
- Ascherio A, Munger KL, White R, Köchert K, Simon KC, Polman CH, et al. Vitamin D as an early predictor of Multiple Sclerosis activity and progression. JAMA Neurol. 2014; 71(3):306-14. [DOI:10.1001/jamaneurol.2013.5993] [PMID] [PMCID]
- Lucas RM, Ponsonby AL. Considering the potential benefits as well as adverse effects of sun exposure: can all the potential benefits be provided by oral vitamin D supplementation? Prog Biophys Mol Biol. 2006; 92(1):140-9. [DOI:10.1016/j.pbiomolbio.2006.02.019] [PMID]
- Antonovsky A, Leibowitz U, Smith HA, Medalie JM, Balogh M, Kats R, et al. Epidemiologic study of Multiple Sclerosis in Israel: I. An overall review of methods and findings. Arch Neurol. 1965; 13(2):183-93. [DOI:10.1001/archneur.1965.00470020073010] [PMID]
- Cendrowski W, Wender M, Dominik W, Flejsierowicz Z, Owsianowski M, Popiel M. Epidemiological study of Multiple Sclerosis in western Poland. Eur Neurol. 1969; 2(2):90-108. [DOI:10.1159/000113777] [PMID]
- Bjørnevik K, Riise T, Casetta I, Drulovic J, Granieri E, Holmøy T, et al. Sun exposure and Multiple Sclerosis risk in Norway and Italy: The EnvIMS study. Mult Scler J. 2014; 20(8):1042-9. [DOI:10.1177/1352458513513968] [PMID]
- Islam T, Gauderman WJ, Cozen W, Mack TM. Childhood sun exposure influences risk of Multiple Sclerosis in monozygotic twins. Neurol. 2007; 69(4):381-8. [DOI:10.1212/01.wnl.0000268266.50850.48] [PMID]
- Tremlett H, Van Der Mei IA, Pittas F, Blizzard L, Paley G, Mesaros D, et al. Monthly ambient sunlight, infections and relapse rates in Multiple Sclerosis. Neuroepidemiology. 2008; 31(4):271-9. [DOI:10.1159/000166602] [PMID]
- Freedman DM, Dosemeci M, Alavanja MC. Mortality from Multiple Sclerosis and exposure to residential and occupational solar radiation: a case-control study based on death certificates. Occup Environ Med. 2000; 57(6):418-21. [DOI:10.1136/oem.57.6.418] [PMID] [PMCID]
Type of Study: Research |
Subject:
Special
Received: 2017/10/10 | Accepted: 2018/03/5 | Published: 2018/07/1
Received: 2017/10/10 | Accepted: 2018/03/5 | Published: 2018/07/1
References
1. Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1, 25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007; 179(3):1634-47. [DOI:10.4049/jimmunol.179.3.1634] [PMID] [DOI:10.4049/jimmunol.179.3.1634]
2. Ciccarelli O, Thompson A. Multiple Sclerosis in 2015: Managing the complexity of Multiple Sclerosis. Nat Rev Neurol. 2016; 12(2):70-2. [DOI:10.1038/nrneurol.2016.2] [PMID] [DOI:10.1038/nrneurol.2016.2]
3. Dutta R, Trapp BD. Relapsing and progressive forms of Multiple Sclerosis–insights from pathology. Curr Opin Neurol. 2014; 27(3):271-8. [DOI:10.1097/WCO.0000000000000094] [PMID] [PMCID] [DOI:10.1097/WCO.0000000000000094]
4. Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sørensen PS, Thompson AJ, et al. Defining the clinical course of Multiple Sclerosis The 2013 revisions. Neurol. 2014; 83(3):278-86. [DOI:10.1212/WNL.0000000000000560] [PMID] [PMCID] [DOI:10.1212/WNL.0000000000000560]
5. Leray E, Moreau T, Fromont A, Edan G. Epidemiology of Multiple Sclerosis. Rev Neurol. 2016; 172(1):3-13. [DOI:10.1016/j.neurol.2015.10.006] [PMID] [DOI:10.1016/j.neurol.2015.10.006]
6. Riccio P, Rossano R. Nutrition facts in Multiple Sclerosis. ASN Neuro. 2015; 7(1):1759091414568185. [DOI:10.1177/1759091414568185] [PMID] [PMCID] [DOI:10.1177/1759091414568185]
7. Riccio P. The molecular basis of nutritional intervention in Multiple Sclerosis: a narrative review. Complement Ther Med. 2011; 19(4):228-37. [DOI:10.1016/j.ctim.2011.06.006] [PMID] [DOI:10.1016/j.ctim.2011.06.006]
8. Ascherio A, Munger KL. Environmental risk factors for Multiple Sclerosis. Part II: Noninfectious factors. Ann Neurol. 2007; 61(6):504-13. [DOI:10.1002/ana.21141] [PMID] [DOI:10.1002/ana.21141]
9. Ascherio A, Munger KL, Simon KC. Vitamin D and Multiple Sclerosis. Lancet Neurol. 2010; 9(6):599-612. [DOI:10.1016/S1474-4422(10)70086-7] [DOI:10.1016/S1474-4422(10)70086-7]
10. Munger KL, Zhang S, O'reilly E, Hernan M, Olek M, Willett W, et al. Vitamin D intake and incidence of Multiple Sclerosis. Neurol. 2004; 62(1):60-5. [DOI:10.1212/01.WNL.0000101723.79681.38] [PMID] [DOI:10.1212/01.WNL.0000101723.79681.38]
11. Muris AH, Smolders J, Rolf L, Klinkenberg LJ, van der Linden N, Meex S, et al. Vitamin D status does not affect disability progression of patients with Multiple Sclerosis over three year follow-up. PloS One. 2016; 11(6):e0156122. [DOI:10.1371/journal.pone.0156122] [PMID] [PMCID] [DOI:10.1371/journal.pone.0156122]
12. Esmael A, El-Sherif M, Elazzouny AA. Effects of vitamin D deficiency on the relapse, severity, and disability of Multiple Sclerosis. Egypt J Neurol Psychiatr Neurosurg. 2016; 53(3):174-8. [DOI:10.4103/1110-1083.193086] [DOI:10.4103/1110-1083.193086]
13. van der Mei IA, Ponsonby AL, Dwyer T, Blizzard L, Simmons R, Taylor B, et al. Past exposure to sun, skin phenotype, and risk of Multiple Sclerosis: Case-control study. Bmj. 2003; 327(7410):316. [DOI:10.1136/bmj.327.7410.316] [PMID] [PMCID] [DOI:10.1136/bmj.327.7410.316]
14. Langer-Gould A, Chen L, Gonzales E, Schroeder C, Xiang A, Barcellos L, et al. Lifetime Sun Exposure and the Risk of Multiple Sclerosis in Blacks and Hispanics (P3. 002). Neurol. 2016; 86(16 Supplement).
15. Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for Multiple Sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011; 69(2):292-302. [DOI:10.1002/ana.22366] [PMID] [PMCID] [DOI:10.1002/ana.22366]
16. Azar M, Sarkisian E. Food composition table of Iran: National Nutrition and Food Research Institute. Tehran: Shahid Beheshti University; 1980.
17. Weihrauch J, Neira P. Provisional table on the vitamin D content of foods. Washington DC: US Department of Agriculture; 1991.
18. Glanz K, Yaroch AL, Dancel M, Saraiya M, Crane LA, Buller DB, et al. Measures of sun exposure and sun protection practices for behavioral and epidemiologic research. Arch Dermatol. 2008; 144(2):217-22. [DOI:10.1001/archdermatol.2007.46] [PMID] [DOI:10.1001/archdermatol.2007.46]
19. Aadahl M, JØrgensen T. Validation of a new self-report instrument for measuring physical activity. Med Sci Sports Exerc. 2003; 35(7):1196-202. [DOI:10.1249/01.MSS.0000074446.02192.14] [PMID] [DOI:10.1249/01.MSS.0000074446.02192.14]
20. Rezazadeh A, Rashidkhani B, Omidvar N. Association of major dietary patterns with socioeconomic and lifestyle factors of adult women living in Tehran, Iran. Nutr. 2010; 26(3):337-41. [DOI:10.1016/j.nut.2009.06.019] [PMID] [DOI:10.1016/j.nut.2009.06.019]
21. Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale: Application to patients with Multiple Sclerosis and systemic lupus erythematosus. Arch Neurol. 1989; 46(10):1121-3. [DOI:10.1001/archneur.1989.00520460115022] [PMID] [DOI:10.1001/archneur.1989.00520460115022]
22. Goldenberg MM. Multiple Sclerosis review. Phys Ther. 2012; 37(3):175-84. [PMID] [PMCID]
23. Braley TJ, Chervin RD. Fatigue in Multiple Sclerosis: Mechanisms, evaluation, and treatment. Sleep. 2010; 33(8):1061. [DOI:10.1093/sleep/33.8.1061] [DOI:10.1093/sleep/33.8.1061]
24. Rottoli M, La Gioia S, Frigeni B, Barcella V. Pathophysiology, assessment and management of Multiple Sclerosis fatigue: An update. Expert Rev Neurother. 2017; 17(4):373-9. [DOI:10.1080/14737175.2017.1247695.] [PMID] [DOI:10.1080/14737175.2017.1247695]
25. Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of Multiple Sclerosis. JAMA. 2006; 296(23):2832-8. [DOI:10.1001/jama.296.23.2832] [PMID] [DOI:10.1001/jama.296.23.2832]
26. Salzer J, Hallmans G, Nyström M, Stenlund H, Wadell G, Sundström P. Vitamin D as a protective factor in Multiple Sclerosis. Neurol. 2012; 79(21):2140-5. [DOI:10.1212/WNL.0b013e3182752ea8] [PMID] [DOI:10.1212/WNL.0b013e3182752ea8]
27. Kampman M, Wilsgaard T, Mellgren S. Outdoor activities and diet in childhood and adolescence relate to MS risk above the Arctic Circle. J Neurol. 2007; 254(4):471-7. [DOI:10.1007/s00415-006-0395-5] [PMID] [DOI:10.1007/s00415-006-0395-5]
28. Mowry EM, Krupp LB, Milazzo M, Chabas D, Strober JB, Belman AL, et al. Vitamin D status is associated with relapse rate in pediatric‐onset Multiple Sclerosis. Ann Neurol. 2010; 67(5):618-24. [PMID] [PMID]
29. Correale J, Ysrraelit MC, Gaitán MI. Immunomodulatory effects of Vitamin D in Multiple Sclerosis. Brain. 2009; 132(5):1146-60. [DOI:10.1093/brain/awp033] [PMID] [DOI:10.1093/brain/awp033]
30. Simpson S, Taylor B, Blizzard L, Ponsonby AL, Pittas F, Tremlett H, et al. Higher 25‐hydroxyvitamin D is associated with lower relapse risk in Multiple Sclerosis. Ann Neurol. 2010; 68(2):193-203. [PMID] [PMID]
31. Soilu-Hänninen M, Airas L, Mononen I, Heikkilä A, Viljanen M, Hänninen A. 25-Hydroxyvitamin D levels in serum at the onset of Multiple Sclerosis. Mult Scler. 2005; 11(3):266-71. [DOI:10.1191/1352458505ms1157oa] [PMID] [DOI:10.1191/1352458505ms1157oa]
32. Smolders J, Menheere P, Kessels A, Damoiseaux J, Hupperts R. Association of vitamin D metabolite levels with relapse rate and disability in Multiple Sclerosis. Mult Scler. 2008; 14(9):1220-4. [DOI:10.1177/1352458508094399] [PMID] [DOI:10.1177/1352458508094399]
33. van der Mei IA, Ponsonby A-L, Dwyer T, Blizzard L, Taylor BV, Kilpatrick T, et al. Vitamin D levels in people with Multiple Sclerosis and community controls in Tasmania, Australia. J Neurol. 2007; 254(5):581. [DOI:10.1007/s00415-006-0315-8] [PMID] [DOI:10.1007/s00415-006-0315-8]
34. Weinstock-Guttman B, Zivadinov R, Qu J, Cookfair D, Duan X, Bang E, et al. Vitamin D metabolites are associated with clinical and MRI outcomes in Multiple Sclerosis patients. J Neurol, Neurosurg & Psychiatry. 2011; 82(2):189-95. [DOI:10.1136/jnnp.2010.227942] [PMID] [DOI:10.1136/jnnp.2010.227942]
35. Ascherio A, Munger KL, White R, Köchert K, Simon KC, Polman CH, et al. Vitamin D as an early predictor of Multiple Sclerosis activity and progression. JAMA Neurol. 2014; 71(3):306-14. [DOI:10.1001/jamaneurol.2013.5993] [PMID] [PMCID] [DOI:10.1001/jamaneurol.2013.5993]
36. Lucas RM, Ponsonby AL. Considering the potential benefits as well as adverse effects of sun exposure: can all the potential benefits be provided by oral vitamin D supplementation? Prog Biophys Mol Biol. 2006; 92(1):140-9. [DOI:10.1016/j.pbiomolbio.2006.02.019] [PMID] [DOI:10.1016/j.pbiomolbio.2006.02.019]
37. Antonovsky A, Leibowitz U, Smith HA, Medalie JM, Balogh M, Kats R, et al. Epidemiologic study of Multiple Sclerosis in Israel: I. An overall review of methods and findings. Arch Neurol. 1965; 13(2):183-93. [DOI:10.1001/archneur.1965.00470020073010] [PMID] [DOI:10.1001/archneur.1965.00470020073010]
38. Cendrowski W, Wender M, Dominik W, Flejsierowicz Z, Owsianowski M, Popiel M. Epidemiological study of Multiple Sclerosis in western Poland. Eur Neurol. 1969; 2(2):90-108. [DOI:10.1159/000113777] [PMID] [DOI:10.1159/000113777]
39. Bjørnevik K, Riise T, Casetta I, Drulovic J, Granieri E, Holmøy T, et al. Sun exposure and Multiple Sclerosis risk in Norway and Italy: The EnvIMS study. Mult Scler J. 2014; 20(8):1042-9. [DOI:10.1177/1352458513513968] [PMID] [DOI:10.1177/1352458513513968]
40. Islam T, Gauderman WJ, Cozen W, Mack TM. Childhood sun exposure influences risk of Multiple Sclerosis in monozygotic twins. Neurol. 2007; 69(4):381-8. [DOI:10.1212/01.wnl.0000268266.50850.48] [PMID] [DOI:10.1212/01.wnl.0000268266.50850.48]
41. Tremlett H, Van Der Mei IA, Pittas F, Blizzard L, Paley G, Mesaros D, et al. Monthly ambient sunlight, infections and relapse rates in Multiple Sclerosis. Neuroepidemiology. 2008; 31(4):271-9. [DOI:10.1159/000166602] [PMID] [DOI:10.1159/000166602]
42. Freedman DM, Dosemeci M, Alavanja MC. Mortality from Multiple Sclerosis and exposure to residential and occupational solar radiation: a case-control study based on death certificates. Occup Environ Med. 2000; 57(6):418-21. [DOI:10.1136/oem.57.6.418] [PMID] [PMCID] [DOI:10.1136/oem.57.6.418]
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |