Thu, Apr 25, 2024
Volume 4, Issue 1 (Winter 2018)
Caspian J Neurol Sci 2018, 4(1): 13-17 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Chitsaz A, Najafi M, Shirmardi M, Mehdipour R. Celecoxib or Prednisolone for Treatment of Medication Overuse Headache: A Randomized, Double-Blind Clinical Trial in Migrainous Patients. Caspian J Neurol Sci 2018; 4 (1) :13-17
URL: http://cjns.gums.ac.ir/article-1-214-en.html
URL: http://cjns.gums.ac.ir/article-1-214-en.html
1- Department of Neurology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
2- Department of Neurology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran , drshirmardi_m@yahoo.com
2- Department of Neurology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran , drshirmardi_m@yahoo.com
Full-Text [PDF 883 kb]
(1112 Downloads)
| Abstract (HTML) (3925 Views)
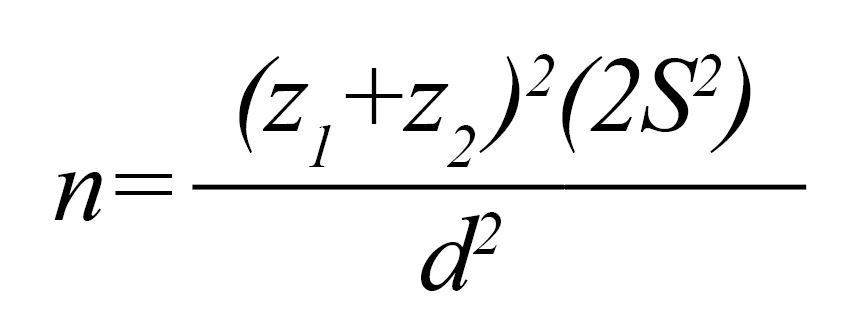
Full-Text: (1248 Views)
Introduction
edication Overuse Headache (MOH) is the second cause of Chronic Daily Headache (CDH). It is a widespread disorder that affects about 1-2% of the general population [1]. The overuse of wide-range medication for migraine and the other types of headache treatment would result in MOH.
In spite of various MOH management recommendations, there is no established consensus on treatment strategies [2]. Prednisolone is recommended by some studies as the standard treatment, but few recently published studies do not suggest so [3]. The first step in MOH management must be withdrawal of the overused drugs and detoxification treatment. Abrupt withdrawal of the medication causing headache. Depending on the medication overused, withdrawal symptoms remain for a 2-to-10-day period, with the average of 3-5 days. Withdrawal treatment normally takes 7 to 14 days [4].
Celecoxib, as a COX-2 inhibitor shows fewer side effects, compared to nonselective anti-inflammatory non-steroidal drugs and corticosteroids, and is not routinely used for headache treatment. The wide-range side effects of prednisolone are convincing enough to let us compare it with a safer drug, with fewer side effects. Since previous studies dealt with headaches with both migraine and tension origins, the aim of this study was to specifically suggest safer medication with higher efficacy and fewer side effects as a replacement therapy for MOH in patients with migraine.
Materials and Methods
Subjects
To reach the aim of the study, we ran a double-blind (patients and the analyzer), parallel-group randomized clinical (prospective) trial. The following formula helped us pick the right sample size, 32 patients:
edication Overuse Headache (MOH) is the second cause of Chronic Daily Headache (CDH). It is a widespread disorder that affects about 1-2% of the general population [1]. The overuse of wide-range medication for migraine and the other types of headache treatment would result in MOH.
In spite of various MOH management recommendations, there is no established consensus on treatment strategies [2]. Prednisolone is recommended by some studies as the standard treatment, but few recently published studies do not suggest so [3]. The first step in MOH management must be withdrawal of the overused drugs and detoxification treatment. Abrupt withdrawal of the medication causing headache. Depending on the medication overused, withdrawal symptoms remain for a 2-to-10-day period, with the average of 3-5 days. Withdrawal treatment normally takes 7 to 14 days [4].
Celecoxib, as a COX-2 inhibitor shows fewer side effects, compared to nonselective anti-inflammatory non-steroidal drugs and corticosteroids, and is not routinely used for headache treatment. The wide-range side effects of prednisolone are convincing enough to let us compare it with a safer drug, with fewer side effects. Since previous studies dealt with headaches with both migraine and tension origins, the aim of this study was to specifically suggest safer medication with higher efficacy and fewer side effects as a replacement therapy for MOH in patients with migraine.
Materials and Methods
Subjects
To reach the aim of the study, we ran a double-blind (patients and the analyzer), parallel-group randomized clinical (prospective) trial. The following formula helped us pick the right sample size, 32 patients:
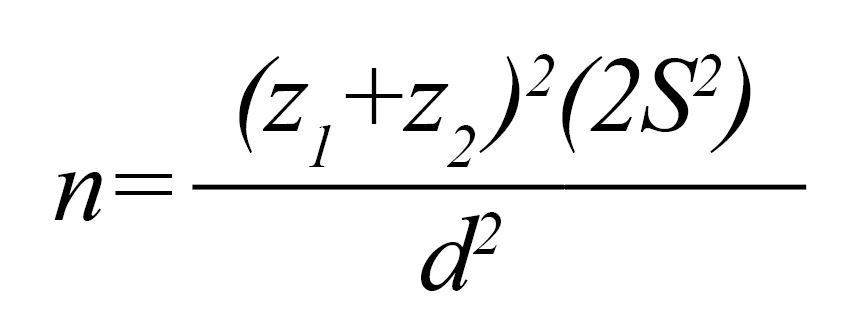
Since some cases may leave the study as the result of the attrition process, we included 4 extra patients (10%). Subjects were selected from those with migraine headaches who visited Neurology Clinic in Isfahan in 2016 in the 18 to 65 age range.
ICD-2 criteria were followed for MOH classification and patient inclusion in the study: 1. Headaches present on more than 15 days/month; 2. Regular overuse for more than 3 months: a) Ergotamine, triptans, opioids, or combination analgesic medications on ≥10 days/month on a regular basis for >3 months; b) Simple analgesic or any combination of ergotamine, triptans, analgesics opioids on ≥15 days/month on a regular basis for >3 months without overuse of any single class alone [5].
Patients with the history of diabetes mellitus, coronary artery disease, psychiatric disorders, those with pregnancy during study, and those who received prophylactic treatment were excluded. Eighty patients with MOH who met the including criteria with balanced block randomization method were assigned into two prednisolone and celecoxib groups. All patients fulfilled the informed consent and could exit from the study any time they wanted or could not tolerate the treatment. This research approved by ethical committee of Isfahan University of Medical Sciences under Code IR.MUI.REC.1395.3.120.
Treatment
One group took oral prednisolone; 75 mg (first 3 days), 50 mg (second 3 days), 25 mg (third 3 days), and 12.5 mg (final 5 days). Patients in another group were prescribed oral celecoxib with the following dosage: 100 mg three times a day (first 5 days), 100 mg twice a day (second 5 days), and 100 mg once a day (final 5 days). Subjects received no prophylactic treatment during the study period. After completing the 15-day period, subjects were interviewed again in terms of the variants of the study, and their possible side effects. We examined any changes in the severity of headaches (by Visual Analogue Scale (VAS) and Migraine Severity (MIGSEV) scale), duration (average hours of daily headache), frequency, and also intake of rescue medication and their side effects of prednisolone and celecoxib carefully. To analyze the data, we ran independent t-test, paired t-test, Mann-Whitney test, Wilcoxon test, and chi-square test. The statistical software was SPSS 20.
Results
Two patients in prednisolone group, and one in celecoxib group were put aside because of medication side effects. In celecoxib group, two patients left the study, due to lack of interest. We finally finished the study with 38 and 37 subjects, respectively, in prednisolone and celecoxib groups. Prednisolone group included subjects with mean age of 36.8±10.3 years (range: 17-60). And celecoxib group included patients with mean age of 34.5±11.5 years (range: 18-63). Independent sample t-test result showed no significant difference between the groups in terms of age (P=0.38). Chi-square test also proved no notable difference in gender frequency distribution of patients between the groups. 13.2% (n=5) in Prednisolone group and 18.9% (n=7) in Celecoxib group were men (P=0.50).
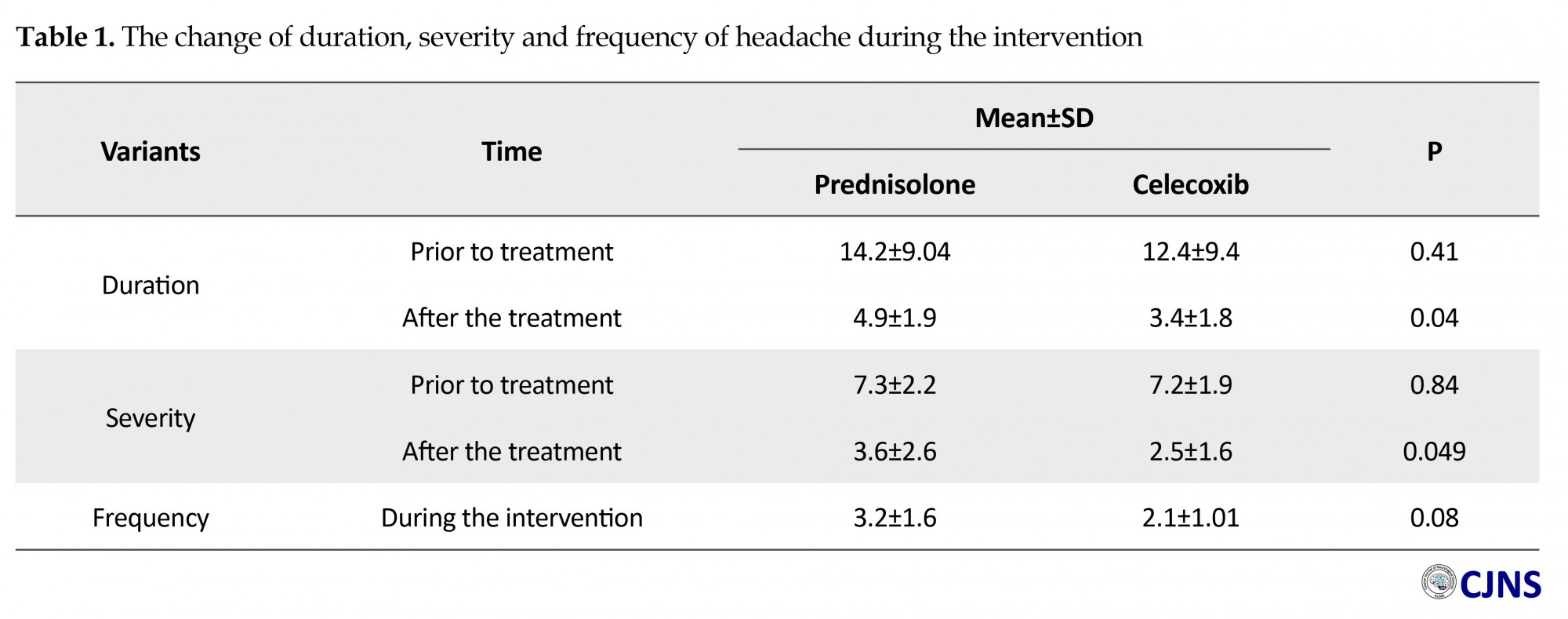
Independent t-test also showed, while the difference of average hours of daily headaches between the groups was not significant prior to the treatment (P=0.41), it significantly decreased in the celecoxib group after the treatment (P=0.04) (Table 1). Additionally, paired sample t-test displayed a significant decrease in average hours of daily headache after the intervention in both groups (P<0.001). Concerning the average of headache intensity score, based on VAS scale, we found no significant difference between the groups prior to the intervention (P=0.84). Nevertheless, it significantly decreased in celecoxib group after the treatment (P=0.049) (Table 1). Paired t-test showed that this average descended noticeably in post intervention period in both groups (P=0.001). Independent t-test indicated no obvious change in headache frequency during the 15-day intervention period between the groups (P=0.08), however, it negligibly was less in the celecoxib group (Table 1).
ICD-2 criteria were followed for MOH classification and patient inclusion in the study: 1. Headaches present on more than 15 days/month; 2. Regular overuse for more than 3 months: a) Ergotamine, triptans, opioids, or combination analgesic medications on ≥10 days/month on a regular basis for >3 months; b) Simple analgesic or any combination of ergotamine, triptans, analgesics opioids on ≥15 days/month on a regular basis for >3 months without overuse of any single class alone [5].
Patients with the history of diabetes mellitus, coronary artery disease, psychiatric disorders, those with pregnancy during study, and those who received prophylactic treatment were excluded. Eighty patients with MOH who met the including criteria with balanced block randomization method were assigned into two prednisolone and celecoxib groups. All patients fulfilled the informed consent and could exit from the study any time they wanted or could not tolerate the treatment. This research approved by ethical committee of Isfahan University of Medical Sciences under Code IR.MUI.REC.1395.3.120.
Treatment
One group took oral prednisolone; 75 mg (first 3 days), 50 mg (second 3 days), 25 mg (third 3 days), and 12.5 mg (final 5 days). Patients in another group were prescribed oral celecoxib with the following dosage: 100 mg three times a day (first 5 days), 100 mg twice a day (second 5 days), and 100 mg once a day (final 5 days). Subjects received no prophylactic treatment during the study period. After completing the 15-day period, subjects were interviewed again in terms of the variants of the study, and their possible side effects. We examined any changes in the severity of headaches (by Visual Analogue Scale (VAS) and Migraine Severity (MIGSEV) scale), duration (average hours of daily headache), frequency, and also intake of rescue medication and their side effects of prednisolone and celecoxib carefully. To analyze the data, we ran independent t-test, paired t-test, Mann-Whitney test, Wilcoxon test, and chi-square test. The statistical software was SPSS 20.
Results
Two patients in prednisolone group, and one in celecoxib group were put aside because of medication side effects. In celecoxib group, two patients left the study, due to lack of interest. We finally finished the study with 38 and 37 subjects, respectively, in prednisolone and celecoxib groups. Prednisolone group included subjects with mean age of 36.8±10.3 years (range: 17-60). And celecoxib group included patients with mean age of 34.5±11.5 years (range: 18-63). Independent sample t-test result showed no significant difference between the groups in terms of age (P=0.38). Chi-square test also proved no notable difference in gender frequency distribution of patients between the groups. 13.2% (n=5) in Prednisolone group and 18.9% (n=7) in Celecoxib group were men (P=0.50).
Independent t-test also showed, while the difference of average hours of daily headaches between the groups was not significant prior to the treatment (P=0.41), it significantly decreased in the celecoxib group after the treatment (P=0.04) (Table 1). Additionally, paired sample t-test displayed a significant decrease in average hours of daily headache after the intervention in both groups (P<0.001). Concerning the average of headache intensity score, based on VAS scale, we found no significant difference between the groups prior to the intervention (P=0.84). Nevertheless, it significantly decreased in celecoxib group after the treatment (P=0.049) (Table 1). Paired t-test showed that this average descended noticeably in post intervention period in both groups (P=0.001). Independent t-test indicated no obvious change in headache frequency during the 15-day intervention period between the groups (P=0.08), however, it negligibly was less in the celecoxib group (Table 1).
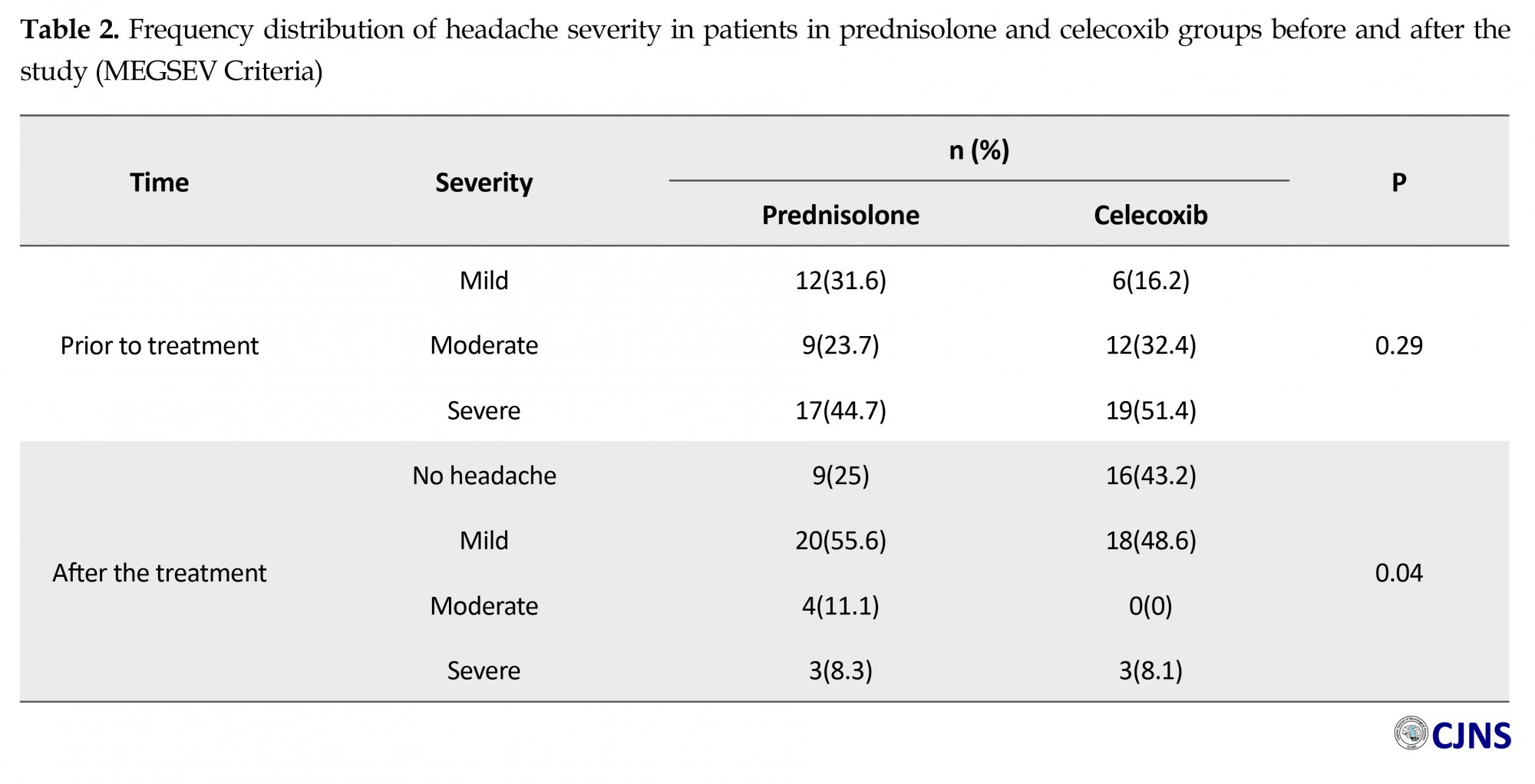
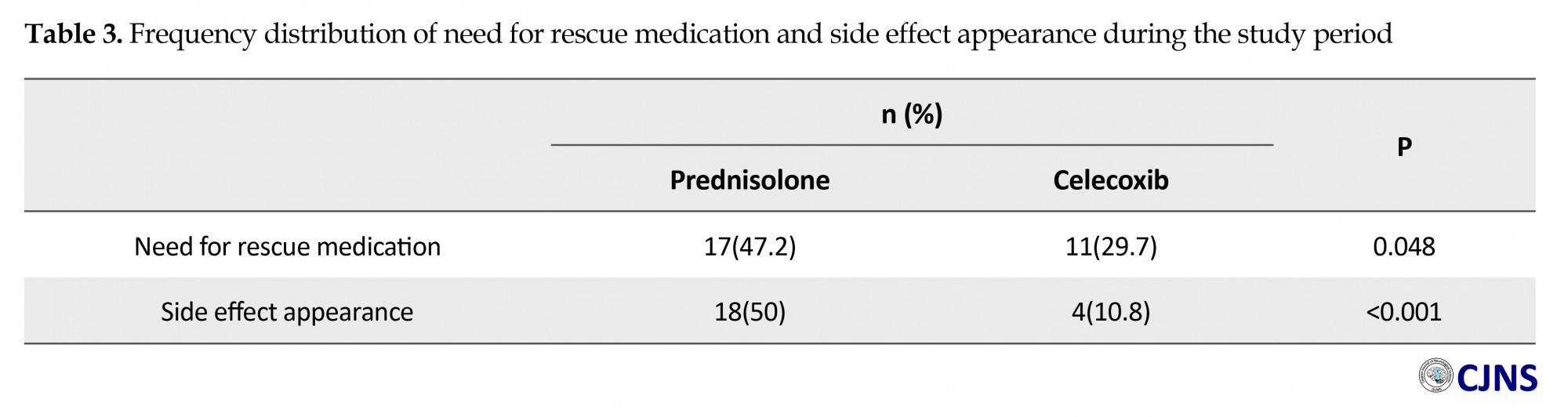
In assessment the change of severity of headache based on MIGSEV scale, Mann-Whitney test showed that although the difference between our groups was ignorable at the beginning (P=0.29), headache severity decreased meaningfully in both groups after the intervention (P<0.001). The bright outcome was the fact that it was much less in celecoxib group (P=0.04) (Table 2). Need for rescue medications (P=0.048), and side effect appearance (P=0.001) was also less in patients who took celecoxib, compared to the other group (Table 3). Table 4 presents further details about the frequency distribution of side effects in post study period.
Discussion
This study was carried out with the aim of investigating and comparing the efficacy of prednisolone and celecoxib, and their possible side effects, in migrainous patients with medication overuse headache. To manage an unbiased study, we ran a double blind experiment. Additionally, P levels, significant statistical differences, and our acceptable sample, all, helped us claim that our results are generalizable to the population of migrainous patients.
Some other studies assessed the management of MOH such as the following reports. Krymchantowski and Moreira (2003) reported an improvement of withdrawal headache following treatment with 60 mg prednisolone orally [3]. Although these results were supported by some studies, Rabe et al. (2013) in a randomized, double-blind, placebo controlled study stated that prednisolone is not effective in the treatment of withdrawal headache in MOH patients and can only reduce the intake of rescue medication during withdrawal [6].
Cevoli et al. (2017) concluded that regardless of the treatment in their population of severe MOH patients, withdrawal headache patients decreased significantly in the 5 days of withdrawal, and neither methylprednisolone nor paracetamol revealed superiority over placebo at the end of the detoxification of the program [7]. Boe et al. (2007) in a clinical trial compared the effect of prednisolone with placebo, and came to the conclusion that prednisolone showed no effect on withdrawal headache in patients with chronic daily headache and medication overuse [8].
Parallel with similar investigations, majority of participants were female (86.6% in prednisolone group, and 81.1% in celecoxib). Average age of subjects in prednisolone and celecoxib was, respectively, 36.8±10.3 and 34.5±11.5. This was also similar to the article by Togha et al. (2014). Another finding was that while the average hours of daily headache was insignificant between the groups prior to the treatment (P=0.41), it was meaningfully lower in the celecoxib group after the treatment (P=0.04). Additionally, we observed a considerable decrease in average hours of daily headache after the intervention in both groups (P<0.001).
Togha et al. (2014) reported the occurrence of daily headaches over 4 hours in both groups, 54% in prednisolone and 35.8% in celecoxib participants. We also came up with the same results concerning the higher efficacy of celecoxib [9]. Concerning the average of headache severity score based on VAS, we found no notable difference between the groups prior to the intervention (P=0.84). However, this item was remarkably lower in celecoxib group after the treatment (P=0.049). It is worth to add that, in both groups, this average descended noticeably in post intervention period (P=0.001) Togha et al. (2014) also claimed the higher efficacy of celecoxib in dealing with headache intensity, however, their achievement was trifling [9].
We also hoped to see an obvious change in headache frequency during the 15-day withdrawal period. Against our wish, and, similar to Togha et al. (2014), our expectation was not met (P=0.08), however, it was negligibly lower in the celecoxib group. 47.2% in prednisolone group, and 29.7% in celecoxib group reported their need for rescue medication during withdrawal. The difference was meaningfully distinguishable (P=0.048). Togha et al. (2014) reported a slightly higher need for rescue medication in prednisolone patients [9].
Headache intensity decreased meaningfully in both groups after the intervention (P<0.001). The other bright outcome was the fact that the intensity was much lower in celecoxib group (P=0.04). The difference between our groups was ignorable at the beginning (P=0.29). Participants were interviewed about any side effect appearance during the program. The side effect appearance was much less in patients who took celecoxib (10.8%), compared to the other group (50%) (P<0.001). Our result was also consistent with earlier researches [4, 9-11].
Togha et al. (2014) [9] claimed that although both prednisolone and celecoxib made contributions to headache alleviation during intervention period, celecoxib had a slight takeover in terms of showing higher efficacy and fewer side effects in patients who developed MOH. While we focused only on patients with migraine headaches, subjects in the study by Togha et al. (2014) [9] suffered from both migrainous and tension headaches.
Conclusion
Considering the findings of our study, we can claim that although both prednisolone and celecoxib showed contribution in reducing headache during withdrawal, celecoxib significantly took over prednisolone in terms of higher efficacy and lower side effects in migrainous patients.
Acknowledgements
This paper is extracted from Maryam Shirmardi's MD thesis in the Department of Neurology, School of Medicine, Isfahan University of Medical Sciences.
Conflict of Interest
The authors have no conflicts of interest.
This study was carried out with the aim of investigating and comparing the efficacy of prednisolone and celecoxib, and their possible side effects, in migrainous patients with medication overuse headache. To manage an unbiased study, we ran a double blind experiment. Additionally, P levels, significant statistical differences, and our acceptable sample, all, helped us claim that our results are generalizable to the population of migrainous patients.
Some other studies assessed the management of MOH such as the following reports. Krymchantowski and Moreira (2003) reported an improvement of withdrawal headache following treatment with 60 mg prednisolone orally [3]. Although these results were supported by some studies, Rabe et al. (2013) in a randomized, double-blind, placebo controlled study stated that prednisolone is not effective in the treatment of withdrawal headache in MOH patients and can only reduce the intake of rescue medication during withdrawal [6].
Cevoli et al. (2017) concluded that regardless of the treatment in their population of severe MOH patients, withdrawal headache patients decreased significantly in the 5 days of withdrawal, and neither methylprednisolone nor paracetamol revealed superiority over placebo at the end of the detoxification of the program [7]. Boe et al. (2007) in a clinical trial compared the effect of prednisolone with placebo, and came to the conclusion that prednisolone showed no effect on withdrawal headache in patients with chronic daily headache and medication overuse [8].
Parallel with similar investigations, majority of participants were female (86.6% in prednisolone group, and 81.1% in celecoxib). Average age of subjects in prednisolone and celecoxib was, respectively, 36.8±10.3 and 34.5±11.5. This was also similar to the article by Togha et al. (2014). Another finding was that while the average hours of daily headache was insignificant between the groups prior to the treatment (P=0.41), it was meaningfully lower in the celecoxib group after the treatment (P=0.04). Additionally, we observed a considerable decrease in average hours of daily headache after the intervention in both groups (P<0.001).
Togha et al. (2014) reported the occurrence of daily headaches over 4 hours in both groups, 54% in prednisolone and 35.8% in celecoxib participants. We also came up with the same results concerning the higher efficacy of celecoxib [9]. Concerning the average of headache severity score based on VAS, we found no notable difference between the groups prior to the intervention (P=0.84). However, this item was remarkably lower in celecoxib group after the treatment (P=0.049). It is worth to add that, in both groups, this average descended noticeably in post intervention period (P=0.001) Togha et al. (2014) also claimed the higher efficacy of celecoxib in dealing with headache intensity, however, their achievement was trifling [9].
We also hoped to see an obvious change in headache frequency during the 15-day withdrawal period. Against our wish, and, similar to Togha et al. (2014), our expectation was not met (P=0.08), however, it was negligibly lower in the celecoxib group. 47.2% in prednisolone group, and 29.7% in celecoxib group reported their need for rescue medication during withdrawal. The difference was meaningfully distinguishable (P=0.048). Togha et al. (2014) reported a slightly higher need for rescue medication in prednisolone patients [9].
Headache intensity decreased meaningfully in both groups after the intervention (P<0.001). The other bright outcome was the fact that the intensity was much lower in celecoxib group (P=0.04). The difference between our groups was ignorable at the beginning (P=0.29). Participants were interviewed about any side effect appearance during the program. The side effect appearance was much less in patients who took celecoxib (10.8%), compared to the other group (50%) (P<0.001). Our result was also consistent with earlier researches [4, 9-11].
Togha et al. (2014) [9] claimed that although both prednisolone and celecoxib made contributions to headache alleviation during intervention period, celecoxib had a slight takeover in terms of showing higher efficacy and fewer side effects in patients who developed MOH. While we focused only on patients with migraine headaches, subjects in the study by Togha et al. (2014) [9] suffered from both migrainous and tension headaches.
Conclusion
Considering the findings of our study, we can claim that although both prednisolone and celecoxib showed contribution in reducing headache during withdrawal, celecoxib significantly took over prednisolone in terms of higher efficacy and lower side effects in migrainous patients.
Acknowledgements
This paper is extracted from Maryam Shirmardi's MD thesis in the Department of Neurology, School of Medicine, Isfahan University of Medical Sciences.
Conflict of Interest
The authors have no conflicts of interest.
References
- Katsarava Z, Diener HC. Medication overuse headache in Germany. Cephalalgia. 2008; 28(11):1221–2. doi: 10.1111/j.1468-2982.2008.01734.x
- Hagen K, Jensen R, Bøe MG, Stovner LJ. Medication overuse headache: A critical review of end points in recent follow-up studies. J Headache Pain. 2010; 11(5):373–7. doi: 10.1007/s10194-010-0221-4
- Krymchantowski A, Moreira P. Out-patient detoxification in chronic migraine: Comparison of strategies. Cephalalgia. 2003; 23(10):982–93. doi: 10.1046/j.1468-2982.2003.00648.x
- Negro A, D’Alonzo L, Martelletti P. Chronic migraine: Comorbidities, risk factors, and rehabilitation. Intern Emerg Med. 2010; 5(S1):13–9. doi: 10.1007/s11739-010-0457-7
- Silberstein SD. Medication overuse headache. New York: American Headache Society; 2008.
- Rabe K, Pageler L, Gaul C, Lampl C, Kraya T, Foerderreuther S, et al. Prednisone for the treatment of withdrawal headache in patients with medication overuse headache: A randomized, double-blind, placebo-controlled study. Cephalalgia. 2012; 33(3):202–7. doi: 10.1177/0333102412462638
- Cevoli S, Giannini G, Favoni V, Terlizzi R, Sancisi E, Nicodemo M, et al. Treatment of withdrawal headache in patients with medication overuse headache: A pilot study. J Headache Pain. 2017; 18(1). doi: 10.1186/s10194-017-0763-9
- Boe MG, Mygland A, Salvesen R. Prednisolone does not reduce withdrawal headache: A randomized, double-blind study. Neurology. 2007; 69(1):26–31. doi: 10.1212/01.wnl.0000263652.46222.e8
- Toghae M, Ghini MR, Paknejad SM, Taghvaii Zahmat Kesh E, Ramim T. Celecoxib versus prednisolone in medication overuse headache withdrawal treatment: double blind randomized clinical trial. Tehran Univ Med J. 2014; 71(12):780-6.
- Krymchantowski A, Barbosa J. Prednisone as initial treatment of analgesic-induced daily headache. Cephalalgia. 2000; 20(2):107–13. doi: 10.1046/j.1468-2982.2000.00028.x
- Grazzi L, Andrasik F, Usai S, Bussone G. In-patient vs. day-hospital withdrawal treatment for chronic migraine with medication overuse and disability assessment: Results at one-year follow-up. Neurol Sci. 2008; 29(S1):161–3. doi: 10.1007/s10072-008-0913-6
Type of Study: Research |
Subject:
Special
Received: 2017/07/3 | Accepted: 2017/10/5 | Published: 2018/01/1
Received: 2017/07/3 | Accepted: 2017/10/5 | Published: 2018/01/1
References
1. Katsarava Z, Diener HC. Medication overuse headache in Germany. Cephalalgia. 2008; 28(11):1221–2. doi: 10.1111/j.1468-2982.2008.01734.x [DOI:10.1111/j.1468-2982.2008.01734.x]
2. Hagen K, Jensen R, Bøe MG, Stovner LJ. Medication overuse headache: A critical review of end points in recent follow-up studies. J Headache Pain. 2010; 11(5):373–7. doi: 10.1007/s10194-010-0221-4 [DOI:10.1007/s10194-010-0221-4]
3. Krymchantowski A, Moreira P. Out-patient detoxification in chronic migraine: Comparison of strategies. Cephalalgia. 2003; 23(10):982–93. doi: 10.1046/j.1468-2982.2003.00648.x [DOI:10.1046/j.1468-2982.2003.00648.x]
4. Negro A, D'Alonzo L, Martelletti P. Chronic migraine: Comorbidities, risk factors, and rehabilitation. Intern Emerg Med. 2010; 5(S1):13–9. doi: 10.1007/s11739-010-0457-7 [DOI:10.1007/s11739-010-0457-7]
5. Silberstein SD. Medication overuse headache. New York: American Headache Society; 2008.
6. Rabe K, Pageler L, Gaul C, Lampl C, Kraya T, Foerderreuther S, et al. Prednisone for the treatment of withdrawal headache in patients with medication overuse headache: A randomized, double-blind, placebo-controlled study. Cephalalgia. 2012; 33(3):202–7. doi: 10.1177/0333102412462638 [DOI:10.1177/0333102412462638]
7. Cevoli S, Giannini G, Favoni V, Terlizzi R, Sancisi E, Nicodemo M, et al. Treatment of withdrawal headache in patients with medication overuse headache: A pilot study. J Headache Pain. 2017; 18(1). doi: 10.1186/s10194-017-0763-9 [DOI:10.1186/s10194-017-0763-9]
8. Boe MG, Mygland A, Salvesen R. Prednisolone does not reduce withdrawal headache: A randomized, double-blind study. Neurology. 2007; 69(1):26–31. doi: 10.1212/01.wnl.0000263652.46222.e8 [DOI:10.1212/01.wnl.0000263652.46222.e8]
9. Toghae M, Ghini MR, Paknejad SM, Taghvaii Zahmat Kesh E, Ramim T. Celecoxib versus prednisolone in medication overuse headache withdrawal treatment: double blind randomized clinical trial. Tehran Univ Med J. 2014; 71(12):780-6.
10. Krymchantowski A, Barbosa J. Prednisone as initial treatment of analgesic-induced daily headache. Cephalalgia. 2000; 20(2):107–13. doi: 10.1046/j.1468-2982.2000.00028.x [DOI:10.1046/j.1468-2982.2000.00028.x]
11. Grazzi L, Andrasik F, Usai S, Bussone G. In-patient vs. day-hospital withdrawal treatment for chronic migraine with medication overuse and disability assessment: Results at one-year follow-up. Neurol Sci. 2008; 29(S1):161–3. doi: 10.1007/s10072-008-0913-6 [DOI:10.1007/s10072-008-0913-6]
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |