Tue, Apr 23, 2024
Volume 4, Issue 4 (Autumn 2018)
Caspian J Neurol Sci 2018, 4(4): 184-189 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Janghorbani M, Barzegar M, Mirmosayyeb O, Shaygannejad V. Ischemic Strokes in a Young Woman With Manifestations of Multiple Sclerosis. Caspian J Neurol Sci 2018; 4 (4) :184-189
URL: http://cjns.gums.ac.ir/article-1-202-en.html
URL: http://cjns.gums.ac.ir/article-1-202-en.html
1- Department of Epidemiology and Biostatistics, School of Health, Isfahan University of Medical Sciences, Isfahan, Iran
2- Student Research Committee, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
3- Isfahan Neurosciences Research Center, Al-Zahra Hospital, Isfahan University of Medical Sciences, Isfahan, Iran , shaygannejad@med.mui.ac.ir
2- Student Research Committee, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
3- Isfahan Neurosciences Research Center, Al-Zahra Hospital, Isfahan University of Medical Sciences, Isfahan, Iran , shaygannejad@med.mui.ac.ir
Full-Text [PDF 1006 kb]
(713 Downloads)
| Abstract (HTML) (2779 Views)
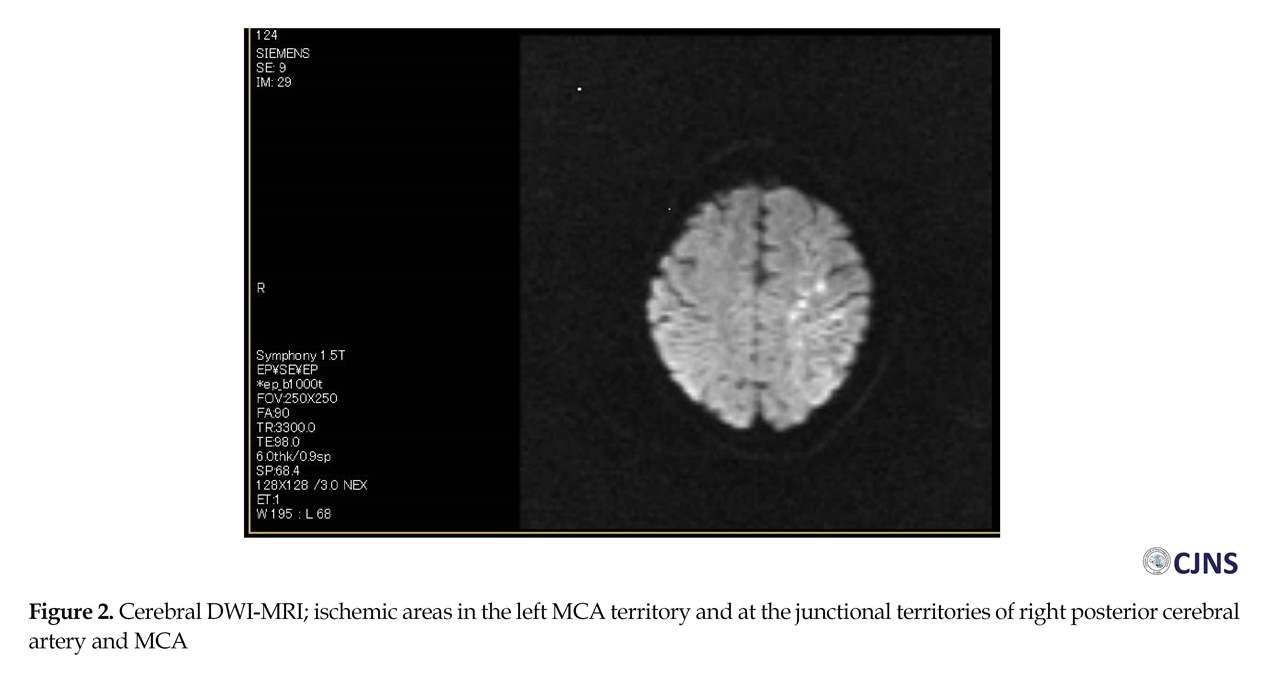
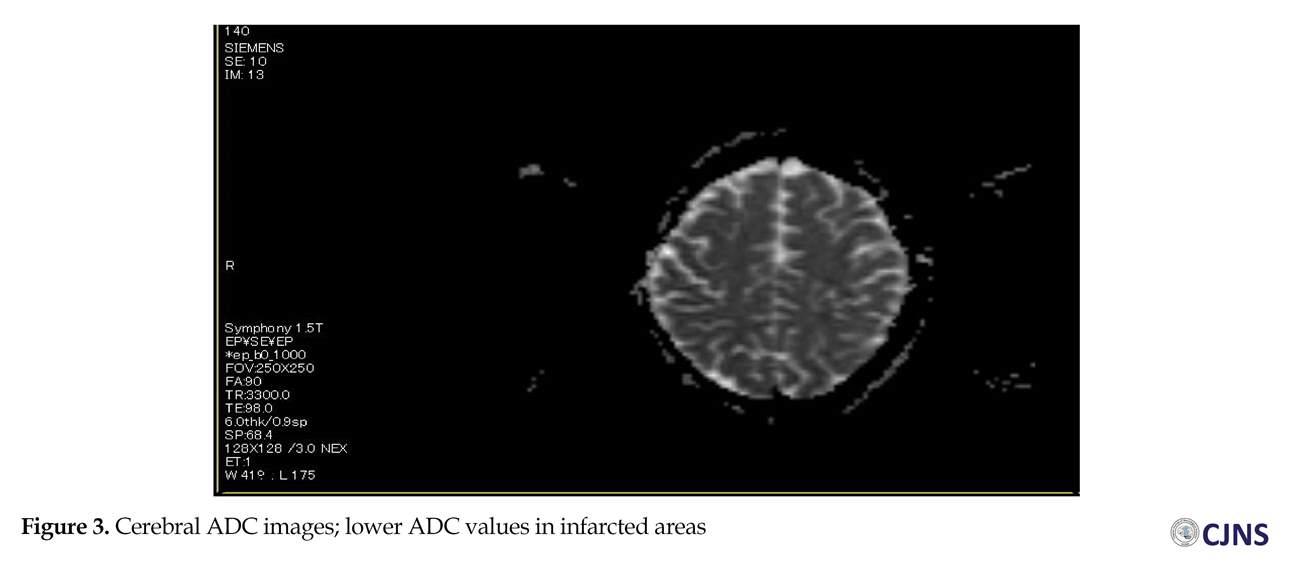
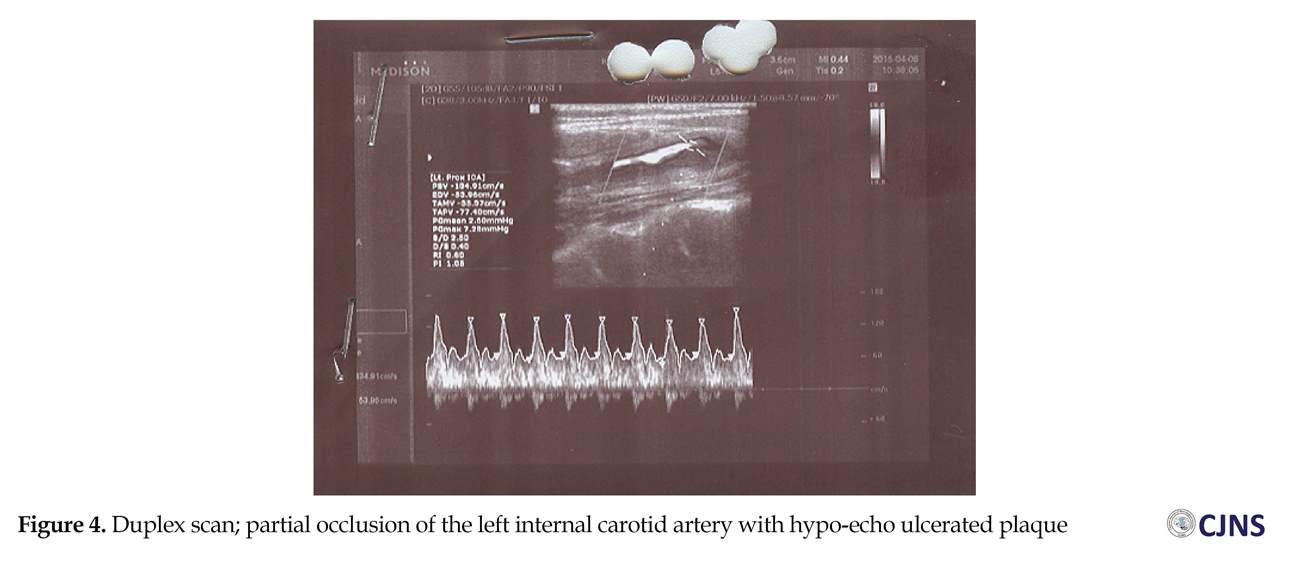
The cerebral Diffusion-Weighted (DW)-MRI detected ischemic areas in subacute state in the left middle cerebral artery territory and the junctional territories of right posterior cerebral artery and middle cerebral artery (Figure 2), with about 25% decline in Apparent Diffusion Coefficient (ADC) values in infarcted areas (Figure 3) and hemorrhagic transformation in the left profound middle cerebral artery area (Figure 4). The partial occlusion of the left internal carotid artery with ulcerated hypo-echo plaque was diagnosed by Duplex scan (Figure 4). There was no evidence of intra or extra-dural mass in cervical MRI.
Tests were positive for Anti-Nuclear Antibody (ANA), Anti-double stain DNA (dsDNA), IgG anti-cardiolipin antibodies, and lupus anticoagulant. Tests were negative for DNA bound lactoferin, Anti-Sm antibodies, Anti-Sjögren’s-Syndrome-related Antigen A (Anti-SSA) autoantibodies, IgM anti-cardiolipin antibodies, and IgG and IgM Anti-beta-2 glycoprotein. There were no symptoms of Systemic Lupus Erythematosus (SLE) or other signs of thrombophilia. The patient was diagnosed with ischemic stroke and multiple watershed infarctions due to microemboli from ulcerated plaque at the left internal carotid artery. Heparin and then warfarin therapy was started. The patient was discharged and referred after two weeks to the Department of Neurology, Kashani Hospital. On admission to the Department of Neurology, clinical characteristics were: BP 130/75 mmHg, heart rate 78 bpm, and sinus rhythm.
Conclusion
In conclusion, based on the patient`s symptoms, at first MS was suspected, but after neurological assessment, ischemic stroke was diagnosed suggesting that ischemic stroke in young patients may be misdiagnosed as an acute form of MS on clinical and imaging examinations. Despite uncertainty concerning the underlying disease mechanism, ischemic stroke can display some acute MS lesions. Neurological assessment and MRI evaluation allow differentiation of the two diseases. This case allowed identifying already known, but also rare, clinical picture in overlap SLE, anti-carliolipin antibody syndrome and ischemic stroke.
Ethical Considerations
Compliance with ethical guidelines
An informed consent was taken from patient before enrollment.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
The authors contributions is as follows: Conceptualization: Mohsen Janghorbani and Mahdi Barzegar; Methodology: All authors; Investigation: Mahdi Barzegar and Omid Mirmosayyeb; Writing–original draft: Mohsen Janghorbani, Mahdi Barzegar and Omid Mirmosayyeb; Writing–review & editing: Vahid Shaygannejad and Mahdi Barzegar; and Supervision: Vahid Shaygannejad.
Conflict of interest
There was no conflict of interest.
References
Full-Text: (788 Views)
Introduction
Multiple Sclerosis (MS) is a common neurological disease often observed in young females [1, 2]. MS is a chronic inflammatory, immune-mediated, debilitating disease that can damage myelin sheaths and the axons of Central Nervous System (CNS) and lead to demyelination and axonal loss [3]. Although successive versions of diagnostic criteria for MS differ in emphasis, MS can be diagnosed based on dissemination of disease in space and time reported by 2010 McDonald criteria [4].
In most patients, with the help of Magnetic Resonance Imaging (MRI) and other investigations, MS can be distinguished from ischemic stroke without difficulty. Occasionally, however, when it presents more acutely, stroke may be misdiagnosed as MS and vice versa. Moreover, MS diagnosis can be made after excluding other diseases that can explain clinical presentation [5, 6]. Immune process in MS disease leads to chronic inflammation that may last a long time [3, 4].
Previous studies showed that chronic inflammation in some inflammatory diseases including rheumatoid arthritis [7, 8], psoriasis [9, 10], and periodontitis [11, 12] cause endothelial dysfunction and accelerate atherosclerosis. This pathway is one of the important reasons of the progression of cardiovascular diseases such as ischemic stroke [13]. Cerebral Watershed Infarction (CWI), also called border zone infarction, is an ischemic lesion that involves the junction between two adjacent arterial territories. The pathophysiology of WI is not clear. Previous investigations found a link between WI and microemboli, from the heart or atherosclerotic artery, which reduces perfusion [14, 15]. The current report presents clinical, radiological, and immunological characteristics of a case with ischemic stroke mimicking, at the onset, MS.
Case Presentation
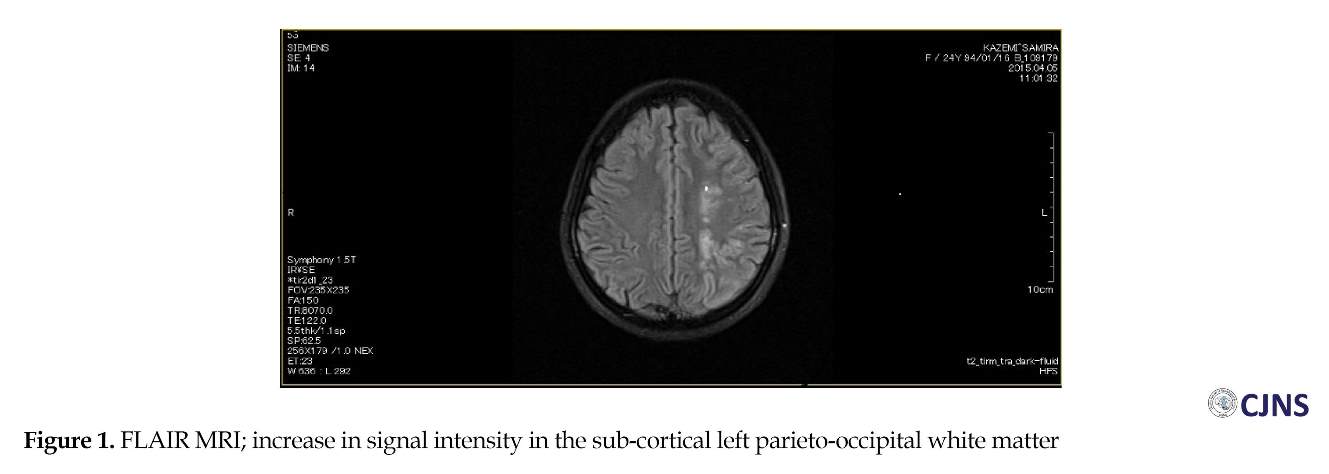
A 24-year old, right-handed Iranian female, taking no treatment, suddenly presented with right lower limb weakness, and was diagnosed with MS by her neurologist and the first Fluid Attenuation Inversion (FLAIR) MRI report (Figure 1). In June 2014, the patient referred to the Emergency Room of the Kashani Hospital affiliated to Isfahan University of Medical Sciences, Isfahan, Iran, with weakness in right lower limb and after four days weakness of the entire right side of her body. A clinical examination revealed right lower facial paresis, reduced muscle tone in the right arm and leg, a right-side impairment of pinprink perception, right hemianopia and Babinski’s sign. Her medical history included intestinal infection when she was three years old with febrile seizure, constipation, and external hemorrhoids.
A cerebral Computed Tomography (CT) scan and MRI was performed; MRI revealed few small and round lesions in deep white matter, semioval centrums, paraventricular region, and subcortical region on left hemisphere. Based on the clinical and radiological findings, MS was diagnosed. The patient was referred to the MS clinic. The neurologic examination was normal, except weakness in right lower limb and psychomotor agitation. During the hospitalization, the neurological status of the patient gradually worsened; after four days, she presented hemiparesis (worsening of weakness in the right lower limb without worsening in right upper limb), dysarthria, hemifacial paresis, and depression.
Multiple Sclerosis (MS) is a common neurological disease often observed in young females [1, 2]. MS is a chronic inflammatory, immune-mediated, debilitating disease that can damage myelin sheaths and the axons of Central Nervous System (CNS) and lead to demyelination and axonal loss [3]. Although successive versions of diagnostic criteria for MS differ in emphasis, MS can be diagnosed based on dissemination of disease in space and time reported by 2010 McDonald criteria [4].
In most patients, with the help of Magnetic Resonance Imaging (MRI) and other investigations, MS can be distinguished from ischemic stroke without difficulty. Occasionally, however, when it presents more acutely, stroke may be misdiagnosed as MS and vice versa. Moreover, MS diagnosis can be made after excluding other diseases that can explain clinical presentation [5, 6]. Immune process in MS disease leads to chronic inflammation that may last a long time [3, 4].
Previous studies showed that chronic inflammation in some inflammatory diseases including rheumatoid arthritis [7, 8], psoriasis [9, 10], and periodontitis [11, 12] cause endothelial dysfunction and accelerate atherosclerosis. This pathway is one of the important reasons of the progression of cardiovascular diseases such as ischemic stroke [13]. Cerebral Watershed Infarction (CWI), also called border zone infarction, is an ischemic lesion that involves the junction between two adjacent arterial territories. The pathophysiology of WI is not clear. Previous investigations found a link between WI and microemboli, from the heart or atherosclerotic artery, which reduces perfusion [14, 15]. The current report presents clinical, radiological, and immunological characteristics of a case with ischemic stroke mimicking, at the onset, MS.
Case Presentation
A 24-year old, right-handed Iranian female, taking no treatment, suddenly presented with right lower limb weakness, and was diagnosed with MS by her neurologist and the first Fluid Attenuation Inversion (FLAIR) MRI report (Figure 1). In June 2014, the patient referred to the Emergency Room of the Kashani Hospital affiliated to Isfahan University of Medical Sciences, Isfahan, Iran, with weakness in right lower limb and after four days weakness of the entire right side of her body. A clinical examination revealed right lower facial paresis, reduced muscle tone in the right arm and leg, a right-side impairment of pinprink perception, right hemianopia and Babinski’s sign. Her medical history included intestinal infection when she was three years old with febrile seizure, constipation, and external hemorrhoids.
A cerebral Computed Tomography (CT) scan and MRI was performed; MRI revealed few small and round lesions in deep white matter, semioval centrums, paraventricular region, and subcortical region on left hemisphere. Based on the clinical and radiological findings, MS was diagnosed. The patient was referred to the MS clinic. The neurologic examination was normal, except weakness in right lower limb and psychomotor agitation. During the hospitalization, the neurological status of the patient gradually worsened; after four days, she presented hemiparesis (worsening of weakness in the right lower limb without worsening in right upper limb), dysarthria, hemifacial paresis, and depression.
The cerebral Diffusion-Weighted (DW)-MRI detected ischemic areas in subacute state in the left middle cerebral artery territory and the junctional territories of right posterior cerebral artery and middle cerebral artery (Figure 2), with about 25% decline in Apparent Diffusion Coefficient (ADC) values in infarcted areas (Figure 3) and hemorrhagic transformation in the left profound middle cerebral artery area (Figure 4). The partial occlusion of the left internal carotid artery with ulcerated hypo-echo plaque was diagnosed by Duplex scan (Figure 4). There was no evidence of intra or extra-dural mass in cervical MRI.
Tests were positive for Anti-Nuclear Antibody (ANA), Anti-double stain DNA (dsDNA), IgG anti-cardiolipin antibodies, and lupus anticoagulant. Tests were negative for DNA bound lactoferin, Anti-Sm antibodies, Anti-Sjögren’s-Syndrome-related Antigen A (Anti-SSA) autoantibodies, IgM anti-cardiolipin antibodies, and IgG and IgM Anti-beta-2 glycoprotein. There were no symptoms of Systemic Lupus Erythematosus (SLE) or other signs of thrombophilia. The patient was diagnosed with ischemic stroke and multiple watershed infarctions due to microemboli from ulcerated plaque at the left internal carotid artery. Heparin and then warfarin therapy was started. The patient was discharged and referred after two weeks to the Department of Neurology, Kashani Hospital. On admission to the Department of Neurology, clinical characteristics were: BP 130/75 mmHg, heart rate 78 bpm, and sinus rhythm.
The neurologic examination showed right homonymous hemianopia, right central facial paresis, right hemiparesis (1/5 MRC), brisk Deep Tendon Reflexes (DTRs), right Babinski’s sign, and dysarthria. The Electrocardiography (ECG) revealed sinus rhythm and QRS axis-60 degrees. Duplex scan of cervico-cerebral arteries evidenced occlusion of left internal carotid artery. Echocardiography examination was normal. Based on clinical and paraclinical (laboratory and imaging) findings, the diagnosis was confirmed: secondary antiphospholipid syndrome, acute thrombosis of the left internal carotid artery, and multiple watershed infarctions in the junctional territory of left posterior and middle cerebral artery. She was currently treated with warfarin, to keep International Normalized Ratio (INR) between 2 and 3, plus hydroxychloroquine (200 mg/d) and atorvastatin (20 mg/d). On the discharge day, the patient’s motor deficit improved (3/5 MRC) and she was able to repeat some words.
Discussion
Ischemic stroke can mimic MS both clinically and on MRI, and cerebral ischemic events can occur as an early manifestation of MS [16]. SLE is also a very complex disease that may involve CNS. The factors contributing to ischemia in SLE are anti-cardiolipin antibodies, other antibodies, atherosclerosis, small vessel vasculopathy, thrombosis, emboli, dissection, vasculitis, vessel spasm, and other risk factors. Both stroke and transient ischemic attacks can occur as an early manifestation of SLE [17] and anti-cardiolipin antibodies are established risk factors for ischemic stroke [18, 19] and SLE [20-25]. The presence of these antibodies increases the risk of cerebral vascular events in patients with immune-mediated disorders [26].
In the first evaluation, the current case was misinterpreted as MS, but after neurological, immunological, and radiological assessments, watershed ischemia was confirmed. Some studies recently show the increased prevalence of ischemic stroke in patients with MS [27-30]. One of the important risk factor for ischemic stroke is age [31-34]. The probability of a coincident ischemic stroke in the patient was considered; but it was unlikely, given that the subsequent clinical and imaging assessment was typical for ischemic stroke.
The current case was erroneously misinterpreted as MS initially. Similar symptoms and imaging of the disease, high incidence of MS in our population, and a low likelihood of ischemic stroke in a very young female patient were the reasons of misdiagnosis.
Discussion
Ischemic stroke can mimic MS both clinically and on MRI, and cerebral ischemic events can occur as an early manifestation of MS [16]. SLE is also a very complex disease that may involve CNS. The factors contributing to ischemia in SLE are anti-cardiolipin antibodies, other antibodies, atherosclerosis, small vessel vasculopathy, thrombosis, emboli, dissection, vasculitis, vessel spasm, and other risk factors. Both stroke and transient ischemic attacks can occur as an early manifestation of SLE [17] and anti-cardiolipin antibodies are established risk factors for ischemic stroke [18, 19] and SLE [20-25]. The presence of these antibodies increases the risk of cerebral vascular events in patients with immune-mediated disorders [26].
In the first evaluation, the current case was misinterpreted as MS, but after neurological, immunological, and radiological assessments, watershed ischemia was confirmed. Some studies recently show the increased prevalence of ischemic stroke in patients with MS [27-30]. One of the important risk factor for ischemic stroke is age [31-34]. The probability of a coincident ischemic stroke in the patient was considered; but it was unlikely, given that the subsequent clinical and imaging assessment was typical for ischemic stroke.
The current case was erroneously misinterpreted as MS initially. Similar symptoms and imaging of the disease, high incidence of MS in our population, and a low likelihood of ischemic stroke in a very young female patient were the reasons of misdiagnosis.
Conclusion
In conclusion, based on the patient`s symptoms, at first MS was suspected, but after neurological assessment, ischemic stroke was diagnosed suggesting that ischemic stroke in young patients may be misdiagnosed as an acute form of MS on clinical and imaging examinations. Despite uncertainty concerning the underlying disease mechanism, ischemic stroke can display some acute MS lesions. Neurological assessment and MRI evaluation allow differentiation of the two diseases. This case allowed identifying already known, but also rare, clinical picture in overlap SLE, anti-carliolipin antibody syndrome and ischemic stroke.
Ethical Considerations
Compliance with ethical guidelines
An informed consent was taken from patient before enrollment.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
The authors contributions is as follows: Conceptualization: Mohsen Janghorbani and Mahdi Barzegar; Methodology: All authors; Investigation: Mahdi Barzegar and Omid Mirmosayyeb; Writing–original draft: Mohsen Janghorbani, Mahdi Barzegar and Omid Mirmosayyeb; Writing–review & editing: Vahid Shaygannejad and Mahdi Barzegar; and Supervision: Vahid Shaygannejad.
Conflict of interest
There was no conflict of interest.
References
- Ahlgren C, Odén A, Lycke J. High nationwide prevalence of multiple Sclerosis in Sweden. Mult Scler J. 2011; 17(8):901-8.[DOI:10.1177/1352458511403794] [PMID]
- Milo R, Kahana E. Multiple Sclerosis: Geoepidemiology, genetics and the environment. Autoimmun Rev. 2010; 9(5):A387-94. [DOI:10.1016/j.autrev.2009.11.010] [PMID]
- Peterson LK, Fujinami RS. Inflammation, demyelination, neurodegeneration and neuroprotection in the pathogenesis of multiple Sclerosis. J Neuroimmunol. 2007; 184(1-2):37-44. [DOI:10.1016/j.jneuroim.2006.11.015] [PMID] [PMCID]
- Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple Sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011; 69(2):292-302. [DOI:10.1002/ana.22366] [PMID] [PMCID]
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple Sclerosis: Guidelines from the International Panel on the diagnosis of Multiple Sclerosis. Ann Neurol. 2001; 50(1):121-27. [DOI:10.1002/ana.1032] [PMID]
- Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple Sclerosis: 2005 revisions to the "McDonald Criteria". Ann Neurol. 2005; 58(6):840-6. [DOI:10.1002/ana.20703] [PMID]
- De Groot L, Posthumus MD, Kallenberg CG, Bijl M. Risk factors and early detection of atherosclerosis in rheumatoid arthritis. Eur J Clin Invest. 2010; 40(9):835-42. [DOI:10.1111/j.1365-2362.2010.02333.x] [PMID]
- Kramer HR, Giles JT. Cardiovascular disease risk in rheumatoid arthritis: progress, debate, and opportunity. Arthritis Care Res. 2011; 63(4):484-99. [DOI:10.1002/acr.20386] [PMID]
- Patel RV, Shelling ML, Prodanovich S, Federman DG, Kirsner RS. Psoriasis and vascular disease-risk factors and outcomes: A systematic review of the literature. Arthritis Care Res. 2011; 26(9):1036-49. [DOI:10.1007/s11606-011-1698-5] [PMID] [PMCID]
- De Simone C, Di Giorgio A, Sisto T, Carbone A, Ghitti F, Tondi P, et al. Endothelial dysfunction in psoriasis patients: Cross-sectional case-control study. Eur J Dermatol. 2011; 21(4):510-4. [PMID]
- Huck O, Saadi Thiers K, Tenenbaum H, Davideau JL, Romagna C, Laurent Y, et al. Evaluating periodontal risk for patients at risk of or suffering from atherosclerosis: Recent biological hypotheses and therapeutic consequences. Arch Cardiovasc Dis. 2011; 104(5):352-8. [DOI:10.1016/j.acvd.2011.02.002] [PMID]
- Beukers NG, Van Der Heijden GJ, Van Wijk AJ, Loos BG. Periodontitis is an independent risk indicator for atherosclerotic cardiovascular diseases among 60 174 participants in a large dental school in the Netherlands. BMJ. 2017; 71; 37-42.
- Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999; 340(2):115-26. [DOI:10.1056/NEJM199901143400207] [PMID]
- Weill C, Suissa L, Darcourt J, Mahagne MH. The pathophysiology of watershed infarction: A three-dimensional time-of-flight magnetic resonance angiography study. J Stroke Cerebrovasc Dis. 2017; 26(9):1966-73. [DOI:10.1016/j.jstrokecerebrovasdis.2017.06.016] [PMID]
- Maki T, Wakita H, Mase M, Itagaki I, Saito N, Ono F, et al. Watershed infarcts in a multiple microembolic model of monkey. Neurosci Lett. 2011; 499(2):80-3. [DOI:10.1016/j.neulet.2011.05.036] [PMID]
- Rosso C, Remy P, Creange A, Brugieres P, Cesaro P, Hosseini H. Diffusion-weighted MR imaging characteristics of acute stroke like form of multiple Sclerosis. AJNR Am J Neuroradiol. 2006; 27(5):1006-8. [PMID]
- Jadidi E, Mohammadi M, Moradi T. High risk of cardiovascular diseases after diagnosis of multiple Sclerosis. Mult Scler J. 2013; 19(10):1336-40. [DOI:10.1177/1352458513475833] [PMID]
- Christiansen CF, Christensen S, Farkas DK, Miret M, Sørensen HT, Pedersen L. Risk of arterial cardiovascular diseases in patients with multiple Sclerosis: A population-based cohort study. Neuroepidemiology. 2010; 35(4):267-74. [DOI:10.1159/000320245] [PMID]
- Marrie RA, Reider N, Cohen J, Stuve O, Trojano M, Cutter G, et al. A systematic review of the incidence and prevalence of cardiac, cerebrovascular, and peripheral vascular disease in multiple Sclerosis. Mult Scler J. 2015; 21(3):318-31. [DOI:10.1177/1352458514564490] [PMID] [PMCID]
- Tseng CH, Huang WS, Lin CL, Chang YJ. Increased risk of ischemic stroke among patients with multiple Sclerosis. Eur J Neurol. 2015; 22(3):500-6. [DOI:10.1111/ene.12598]
- Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008; 371(9624):1612-23. [DOI:10.1016/S0140-6736(08)60694-7]
- Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011; 42(2):517-84. [DOI:10.1161/STR.0b013e3181fcb238] [PMID]
- The American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2010 update: A report from the American Heart Association. Circulation. 2010; 121(7):e46-e215. [PMID]
- Harmsen P, Lappas G, Rosengren A, Wilhelmsen L. Long-term risk factors for stroke: Twenty-eight years of follow-up of 7457 middle-aged men in Göteborg, Sweden. Stroke. 2006; 37(7):1663-7. [DOI:10.1161/01.STR.0000226604.10877.fc] [PMID]
- Haas LF. Stroke as an early manifestation of systemic lupus erythematosus. Journal of Neurology, Neurosurgery & Psychiatry. 1982; 45(6):554-6. [DOI:10.1136/jnnp.45.6.554] [PMID] [PMCID]
- Neville C, Rauch J, Kassis J, Solymoss S, Joseph L, Belisle P, et al. Antiphospholipid antibodies predict imminent vascular events independently from other risk factors in a prospective cohort. Thrombosis and Haemostasis. 2009; 101(1):100-7. [DOI:10.1160/TH08-06-0384] [PMID] [PMCID]
- Brey RL, Muscal E, Chapman J. Antiphospholipid antibodies and the brain: A consensus report. Lupus. 2011; 20(2):153-7. [DOI:10.1177/0961203310396748] [PMID]
- Sebastiani GD, Galeazzi M, Tincani A, Piette JC, Font J, Allegri F, et al. Anticardiolipin and anti-β2GPI antibodies in a large series of European patients with systemic lupus erythematosus. Scandinavian Journal of Rheumatology. 1999; 28(6):344-51. [DOI:10.1080/03009749950155320] [PMID]
- Soltesz P, Veres K, Lakos G, Kiss E, Muszbek L, Szegedi G. Evaluation of clinical and laboratory features of antiphospholipid syndrome: A retrospective study of 637 patients. Lupus. 2003; 12(4):302-7. [DOI:10.1191/0961203303lu339oa] [PMID]
- Alarc N, Segovia D, Deleze M, Oria CV. Antiphospholipid antibodies and the antiphospholipid syndrome in systemic lupus erythematosus. A prospective analysis of 500 consecutive patients. Med. 1989; 68(6):353–65. [DOI:10.1097/00005792-198911000-00003]
- Love PE, Santoro SA. Antiphospholipid antibodies: Anticardiolipin and the lupus anticoagulant in Systemic Lupus Erythematosus (SLE) and in non SLE disorders. Ann Intern Med. 1990; 112(9):682–98. [DOI:10.7326/0003-4819-112-9-682] [PMID]
- Remondino G, Allevi A. Antiphospholipid antibodies and autoimmune diseases. Open Autoimmun J. 2010; 2:38-44. [DOI:10.2174/1876894601002020038]
- Tarr T, Lakos G, Bhattoa HP, Szegedi G, Shoenfeld Y, Kiss E. Primary antiphospholipid syndrome as the forerunner of systemic lupus erythematosus. Lupus. 2007; 16(5):324-9. [DOI:10.1177/0961203307077993] [PMID]
- Tanasescu R, Nicolau A, Ticmeanu M, Cojocaru I, Ene A, Caraiola S, et al. Headache, antiphospholipid antibodies and cerebral ischemia in patients with systemic immune-mediated diseases. Rom J Neurol. 2007; 6(2):71-108.
Type of Study: case report |
Subject:
Special
Received: 2018/01/5 | Accepted: 2018/06/13 | Published: 2018/10/1
Received: 2018/01/5 | Accepted: 2018/06/13 | Published: 2018/10/1
References
1. Ahlgren C, Odén A, Lycke J. High nationwide prevalence of multiple Sclerosis in Sweden. Mult Scler J. 2011; 17(8):901-8.[DOI:10.1177/1352458511403794] [PMID] [DOI:10.1177/1352458511403794]
2. Milo R, Kahana E. Multiple Sclerosis: Geoepidemiology, genetics and the environment. Autoimmun Rev. 2010; 9(5):A387-94. [DOI:10.1016/j.autrev.2009.11.010] [PMID] [DOI:10.1016/j.autrev.2009.11.010]
3. Peterson LK, Fujinami RS. Inflammation, demyelination, neurodegeneration and neuroprotection in the pathogenesis of multiple Sclerosis. J Neuroimmunol. 2007; 184(1-2):37-44. [DOI:10.1016/j.jneuroim.2006.11.015] [PMID] [PMCID] [DOI:10.1016/j.jneuroim.2006.11.015]
4. Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple Sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011; 69(2):292-302. [DOI:10.1002/ana.22366] [PMID] [PMCID] [DOI:10.1002/ana.22366]
5. McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple Sclerosis: Guidelines from the International Panel on the diagnosis of Multiple Sclerosis. Ann Neurol. 2001; 50(1):121-27. [DOI:10.1002/ana.1032] [PMID] [DOI:10.1002/ana.1032]
6. Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple Sclerosis: 2005 revisions to the "McDonald Criteria". Ann Neurol. 2005; 58(6):840-6. [DOI:10.1002/ana.20703] [PMID] [DOI:10.1002/ana.20703]
7. De Groot L, Posthumus MD, Kallenberg CG, Bijl M. Risk factors and early detection of atherosclerosis in rheumatoid arthritis. Eur J Clin Invest. 2010; 40(9):835-42. [DOI:10.1111/j.1365-2362.2010.02333.x] [PMID] [DOI:10.1111/j.1365-2362.2010.02333.x]
8. Kramer HR, Giles JT. Cardiovascular disease risk in rheumatoid arthritis: progress, debate, and opportunity. Arthritis Care Res. 2011; 63(4):484-99. [DOI:10.1002/acr.20386] [PMID] [DOI:10.1002/acr.20386]
9. Patel RV, Shelling ML, Prodanovich S, Federman DG, Kirsner RS. Psoriasis and vascular disease-risk factors and outcomes: A systematic review of the literature. Arthritis Care Res. 2011; 26(9):1036-49. [DOI:10.1007/s11606-011-1698-5] [PMID] [PMCID] [DOI:10.1007/s11606-011-1698-5]
10. De Simone C, Di Giorgio A, Sisto T, Carbone A, Ghitti F, Tondi P, et al. Endothelial dysfunction in psoriasis patients: Cross-sectional case-control study. Eur J Dermatol. 2011; 21(4):510-4. [PMID] [PMID]
11. Huck O, Saadi Thiers K, Tenenbaum H, Davideau JL, Romagna C, Laurent Y, et al. Evaluating periodontal risk for patients at risk of or suffering from atherosclerosis: Recent biological hypotheses and therapeutic consequences. Arch Cardiovasc Dis. 2011; 104(5):352-8. [DOI:10.1016/j.acvd.2011.02.002] [PMID] [DOI:10.1016/j.acvd.2011.02.002]
12. Beukers NG, Van Der Heijden GJ, Van Wijk AJ, Loos BG. Periodontitis is an independent risk indicator for atherosclerotic cardiovascular diseases among 60 174 participants in a large dental school in the Netherlands. BMJ. 2017; 71; 37-42.
13. Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med. 1999; 340(2):115-26. [DOI:10.1056/NEJM199901143400207] [PMID] [DOI:10.1056/NEJM199901143400207]
14. Weill C, Suissa L, Darcourt J, Mahagne MH. The pathophysiology of watershed infarction: A three-dimensional time-of-flight magnetic resonance angiography study. J Stroke Cerebrovasc Dis. 2017; 26(9):1966-73. [DOI:10.1016/j.jstrokecerebrovasdis.2017.06.016] [PMID] [DOI:10.1016/j.jstrokecerebrovasdis.2017.06.016]
15. Maki T, Wakita H, Mase M, Itagaki I, Saito N, Ono F, et al. Watershed infarcts in a multiple microembolic model of monkey. Neurosci Lett. 2011; 499(2):80-3. [DOI:10.1016/j.neulet.2011.05.036] [PMID] [DOI:10.1016/j.neulet.2011.05.036]
16. Rosso C, Remy P, Creange A, Brugieres P, Cesaro P, Hosseini H. Diffusion-weighted MR imaging characteristics of acute stroke like form of multiple Sclerosis. AJNR Am J Neuroradiol. 2006; 27(5):1006-8. [PMID] [PMID]
17. Jadidi E, Mohammadi M, Moradi T. High risk of cardiovascular diseases after diagnosis of multiple Sclerosis. Mult Scler J. 2013; 19(10):1336-40. [DOI:10.1177/1352458513475833] [PMID] [DOI:10.1177/1352458513475833]
18. Christiansen CF, Christensen S, Farkas DK, Miret M, Sørensen HT, Pedersen L. Risk of arterial cardiovascular diseases in patients with multiple Sclerosis: A population-based cohort study. Neuroepidemiology. 2010; 35(4):267-74. [DOI:10.1159/000320245] [PMID] [DOI:10.1159/000320245]
19. Marrie RA, Reider N, Cohen J, Stuve O, Trojano M, Cutter G, et al. A systematic review of the incidence and prevalence of cardiac, cerebrovascular, and peripheral vascular disease in multiple Sclerosis. Mult Scler J. 2015; 21(3):318-31. [DOI:10.1177/1352458514564490] [PMID] [PMCID] [DOI:10.1177/1352458514564490]
20. Tseng CH, Huang WS, Lin CL, Chang YJ. Increased risk of ischemic stroke among patients with multiple Sclerosis. Eur J Neurol. 2015; 22(3):500-6. [DOI:10.1111/ene.12598] [DOI:10.1111/ene.12598]
21. Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008; 371(9624):1612-23. [DOI:10.1016/S0140-6736(08)60694-7] [DOI:10.1016/S0140-6736(08)60694-7]
22. Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011; 42(2):517-84. [DOI:10.1161/STR.0b013e3181fcb238] [PMID] [DOI:10.1161/STR.0b013e3181fcb238]
23. The American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2010 update: A report from the American Heart Association. Circulation. 2010; 121(7):e46-e215. [PMID] [PMID]
24. Harmsen P, Lappas G, Rosengren A, Wilhelmsen L. Long-term risk factors for stroke: Twenty-eight years of follow-up of 7457 middle-aged men in Göteborg, Sweden. Stroke. 2006; 37(7):1663-7. [DOI:10.1161/01.STR.0000226604.10877.fc] [PMID] [DOI:10.1161/01.STR.0000226604.10877.fc]
25. Haas LF. Stroke as an early manifestation of systemic lupus erythematosus. Journal of Neurology, Neurosurgery & Psychiatry. 1982; 45(6):554-6. [DOI:10.1136/jnnp.45.6.554] [PMID] [PMCID] [DOI:10.1136/jnnp.45.6.554]
26. Neville C, Rauch J, Kassis J, Solymoss S, Joseph L, Belisle P, et al. Antiphospholipid antibodies predict imminent vascular events independently from other risk factors in a prospective cohort. Thrombosis and Haemostasis. 2009; 101(1):100-7. [DOI:10.1160/TH08-06-0384] [PMID] [PMCID] [DOI:10.1160/TH08-06-0384]
27. Brey RL, Muscal E, Chapman J. Antiphospholipid antibodies and the brain: A consensus report. Lupus. 2011; 20(2):153-7. [DOI:10.1177/0961203310396748] [PMID] [DOI:10.1177/0961203310396748]
28. Sebastiani GD, Galeazzi M, Tincani A, Piette JC, Font J, Allegri F, et al. Anticardiolipin and anti-β2GPI antibodies in a large series of European patients with systemic lupus erythematosus. Scandinavian Journal of Rheumatology. 1999; 28(6):344-51. [DOI:10.1080/03009749950155320] [PMID] [DOI:10.1080/03009749950155320]
29. Soltesz P, Veres K, Lakos G, Kiss E, Muszbek L, Szegedi G. Evaluation of clinical and laboratory features of antiphospholipid syndrome: A retrospective study of 637 patients. Lupus. 2003; 12(4):302-7. [DOI:10.1191/0961203303lu339oa] [PMID] [DOI:10.1191/0961203303lu339oa]
30. Alarc N, Segovia D, Deleze M, Oria CV. Antiphospholipid antibodies and the antiphospholipid syndrome in systemic lupus erythematosus. A prospective analysis of 500 consecutive patients. Med. 1989; 68(6):353–65. [DOI:10.1097/00005792-198911000-00003] [DOI:10.1097/00005792-198911000-00003]
31. Love PE, Santoro SA. Antiphospholipid antibodies: Anticardiolipin and the lupus anticoagulant in Systemic Lupus Erythematosus (SLE) and in non SLE disorders. Ann Intern Med. 1990; 112(9):682–98. [DOI:10.7326/0003-4819-112-9-682] [PMID] [DOI:10.7326/0003-4819-112-9-682]
32. Remondino G, Allevi A. Antiphospholipid antibodies and autoimmune diseases. Open Autoimmun J. 2010; 2:38-44. [DOI:10.2174/1876894601002020038] [DOI:10.2174/1876894601002020038]
33. Tarr T, Lakos G, Bhattoa HP, Szegedi G, Shoenfeld Y, Kiss E. Primary antiphospholipid syndrome as the forerunner of systemic lupus erythematosus. Lupus. 2007; 16(5):324-9. [DOI:10.1177/0961203307077993] [PMID] [DOI:10.1177/0961203307077993]
34. Tanasescu R, Nicolau A, Ticmeanu M, Cojocaru I, Ene A, Caraiola S, et al. Headache, antiphospholipid antibodies and cerebral ischemia in patients with systemic immune-mediated diseases. Rom J Neurol. 2007; 6(2):71-108.
Send email to the article author
| Rights and permissions | |
 | This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |